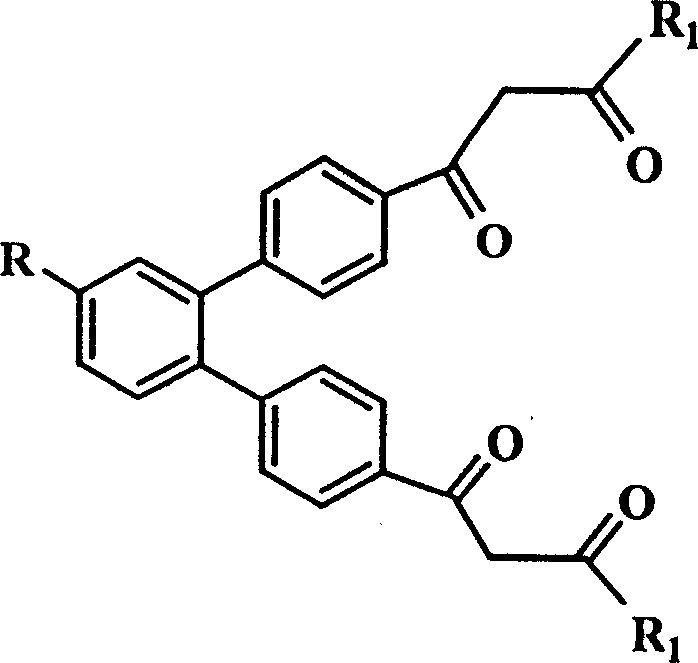
Trivalent europium-beta-diketone fluorescent label and uses thereof
A fluorescent marker, trivalent europium technology, used in biological testing, material testing products, measuring devices, etc., can solve the problems of expensive reagents and low sensitivity, and achieve low cost, high sensitivity, and simple reaction and separation operations. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
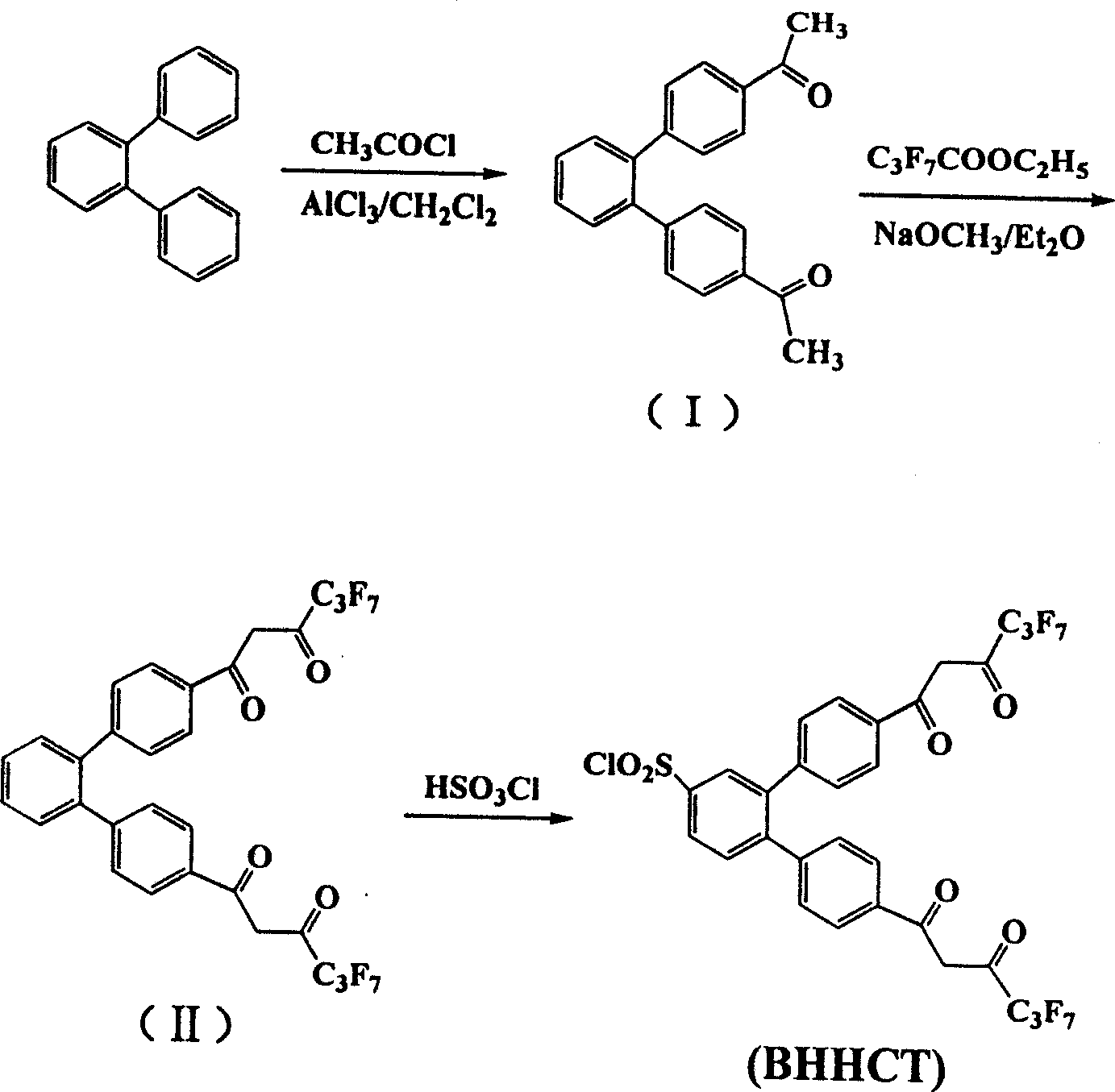
[0038] Synthesis of 4-dentate β-diketone ligands
[0039] (1) 4,4'-bis(1",1",1",2",2",3",3"-heptafluoro-4",6"-hexanedione-6"-yl)chloride Synthesis of sulfonyl-o-diphenylbenzene (abbreviated as BHHCT). BHHCT press figure 2 The synthetic route shown is synthesized, and the specific operation process is as follows.
[0040] (i) Synthesis of 4,4'-Diacetyl-o-diphenylbenzene (Compound I)
[0041] Under external ice-water bath cooling, dissolve 14 g of anhydrous aluminum trichloride and 8.1 g of acetyl chloride in 100 ml of dry dichloromethane, and add 11.5 g of o-diphenylbenzene in 50 ml of dichloromethane with stirring. The solution. The reaction solution was stirred for 30 minutes under cooling in an ice-water bath, then stirred at room temperature for 24 hours, and then back distilled for another 2 hours. The reaction solution was poured into a mixture of ice and hydrochloric acid, and after dichloromethane was distilled off, the precipitate was collected by filtration. After the pr...
Embodiment 2
[0051] Use BHHCT-Eu 3+ Marker protein
[0052] (1) Use BHHCT-Eu 3+ Mark BSA
[0053] Dissolve 5 mg of BSA in 1 ml of 0.1 mol / L sodium bicarbonate buffer solution with a pH of 9.3, and add 3.5 mg of BHHCT in 200 microliters of DMF dropwise with stirring. After stirring for 1 hour at room temperature, the labeled BSA and unreacted BHHCT were separated using Sephadex G-50 gel column, and developed with 0.05 mol / L ammonium bicarbonate solution. Measure the absorbance of the labeled BSA solution at 330nm, and use the molar absorption coefficient of BHHCT at 330nm (3.41×10 4 cm -1 mol -1 L) Calculate the concentration of BHHCT in the labeled BSA solution, and then calculate the labeling rate (the ratio of the concentration of BHHCT to the concentration of BSA). The labeling rate of labeled BSA prepared by this method is 35. Add EuCl equal to BHHCT in the solution 3 After that, the fluorescent labeled BSA solution BSA (BHHCT-Eu 3+ ) 35 . Add NaN to the solution 3 (0.1%) After adjusting t...
Embodiment 3
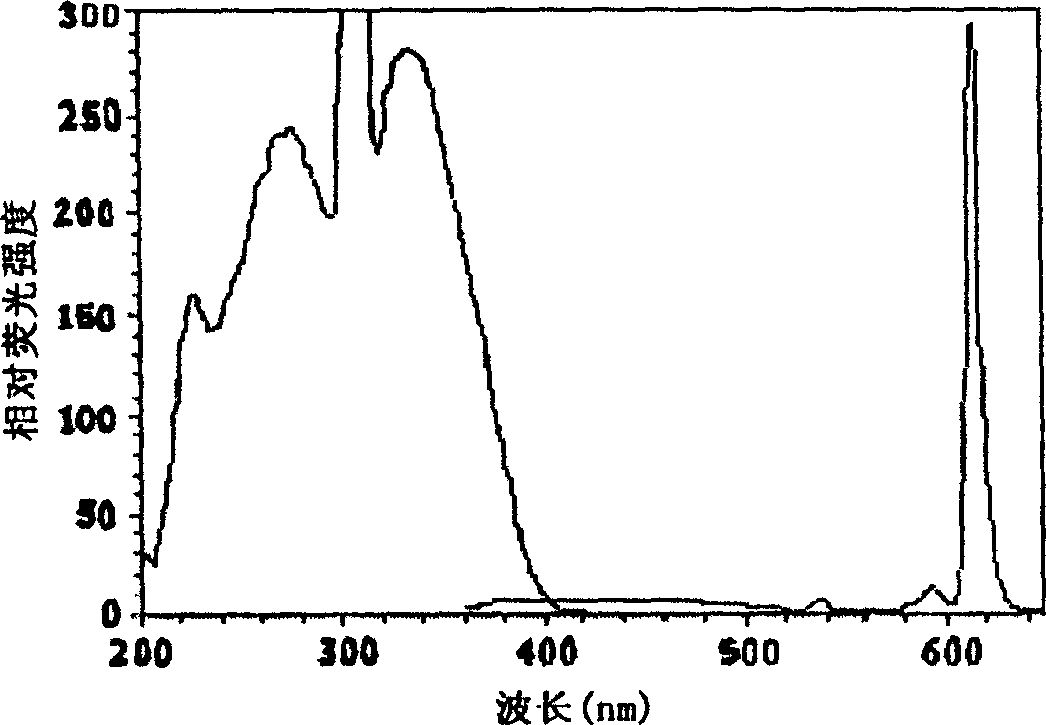
[0061] BHHCT-Eu 3+ Fluorescence properties of labeled BSA solution
[0062] Such as image 3 As shown, BHHCT-Eu 3+ The maximum fluorescence excitation wavelength is 330nm, the maximum emission wavelength is 614nm, and its emission peak shape is a sharp emission peak characteristic of europium complexes. Use BHHCT-Eu 3+ BHHCT-Eu measured with BSA solution 3+ In the 0.05mol / L Tris-HCl buffer solution with pH 7.8, the fluorescence lifetime is 380 microseconds, the fluorescence quantum yield is 0.27, and it contains 1.0×10 -5 The fluorescence lifetime in a 0.1 mol / L sodium carbonate solution of mol / L trioctyl phosphorus oxide and 0.05% sodium dodecyl sulfonate is 641 microseconds, and the fluorescence quantum yield is 0.76.
[0063] BHHCT-Eu 3+ The time-resolved fluorescence measurement results of the labeled BSA serial dilution solution are as follows Figure 4 As shown, using 2 times the standard deviation of the background signal to calculate the lowest detection limit, BSA (BHHCT-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com