Controlling T7 expression system by means of thermal induction
An expression system and heat-induced technology, applied in biochemical equipment and methods, using vectors to introduce foreign genetic material, DNA/RNA fragments, etc., can solve the problems of unreachable industrial use, instability, high price, etc., and achieve simple and economical competitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment ( 1
[0034] Embodiment (1), construction of recombinant strain BL21 (G2)
[0035] In order to develop a heat-inducible way to control the expression system of T7, firstly, a recombinant strain will be constructed so that its chromosome contains a lambda P L and P R Dual-promoter regulated T7 gene 1 and a heat-sensitive cI857 repressor gene.
[0036] A. Cloning operation of T7 gene 1
[0037] (1) Bacteria used for gene cloning and shake flask culture method
[0038] Escherichia coli DH5 α (deoR endA1 gyr96 hsdR17supE444 thiΔ(lacIZYA-argF 169) recA1 lacZM15) was used in the gene cloning process. When cultivating strains in liquid state, firstly, a few colonies were picked from the solid petri dish to the shake flask containing nutrient medium Culture overnight at 30°C in a water bath rotating culture tank at 200 revolutions per minute, take out the bacterial solution in a hundred-fold diluted volume the next day and inoculate it into a shaker flask containing fresh nutrient matrix...
Embodiment ( 2
[0048] Embodiment (two), carry out the detection pattern of shaking flask scale with strain BL21 (G2)
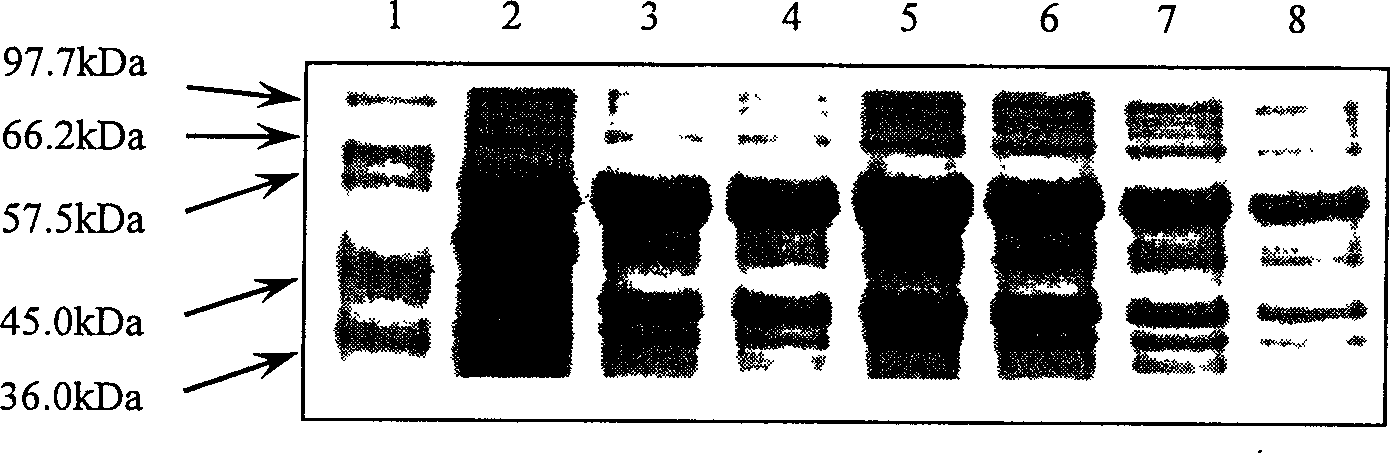
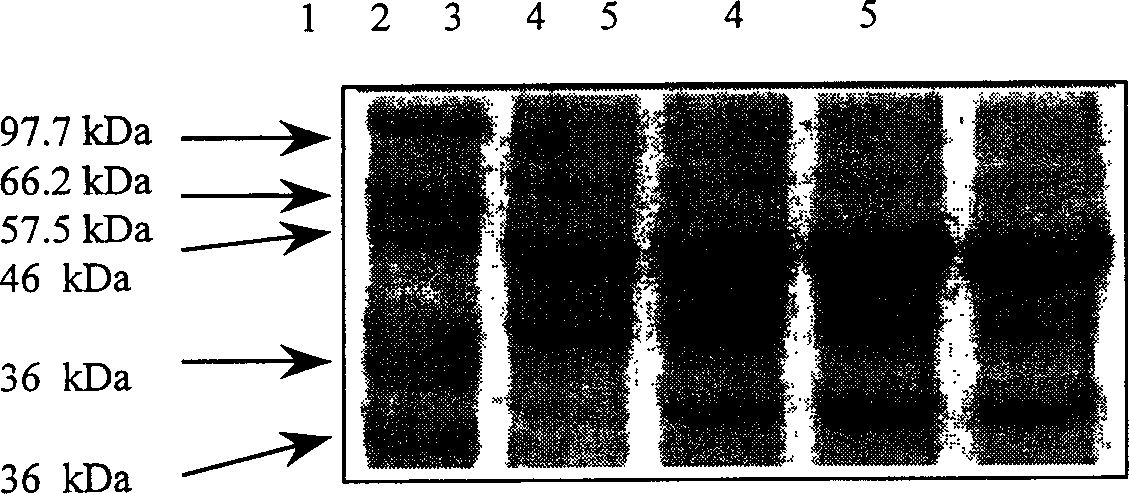
[0049] To test the feasibility of recombinant strain BL21(G2) producing heterologous recombinant protein, here we choose the production of carbamoylase as the detection mode. The biggest challenge in the production of heterologous protein is that the protein is easy to form inclusion bodies and has the characteristics of poisoning cells, and the carbamylase from A.radiobacter is a heterologous protein, and it is very easy to form inclusion bodies body and potential toxicity, so this protein as a production model is sufficient to provide the most stringent detection method for the system constructed in the present invention.
[0050] A. Training method
[0051] The same as the cultivation method of item A in the construction of the recombinant strain BL21 (G2), use shake flasks to cultivate the strain.
[0052] B. Carbamylase Enzyme Activity Analysis
[0053] The analysis ...
Embodiment
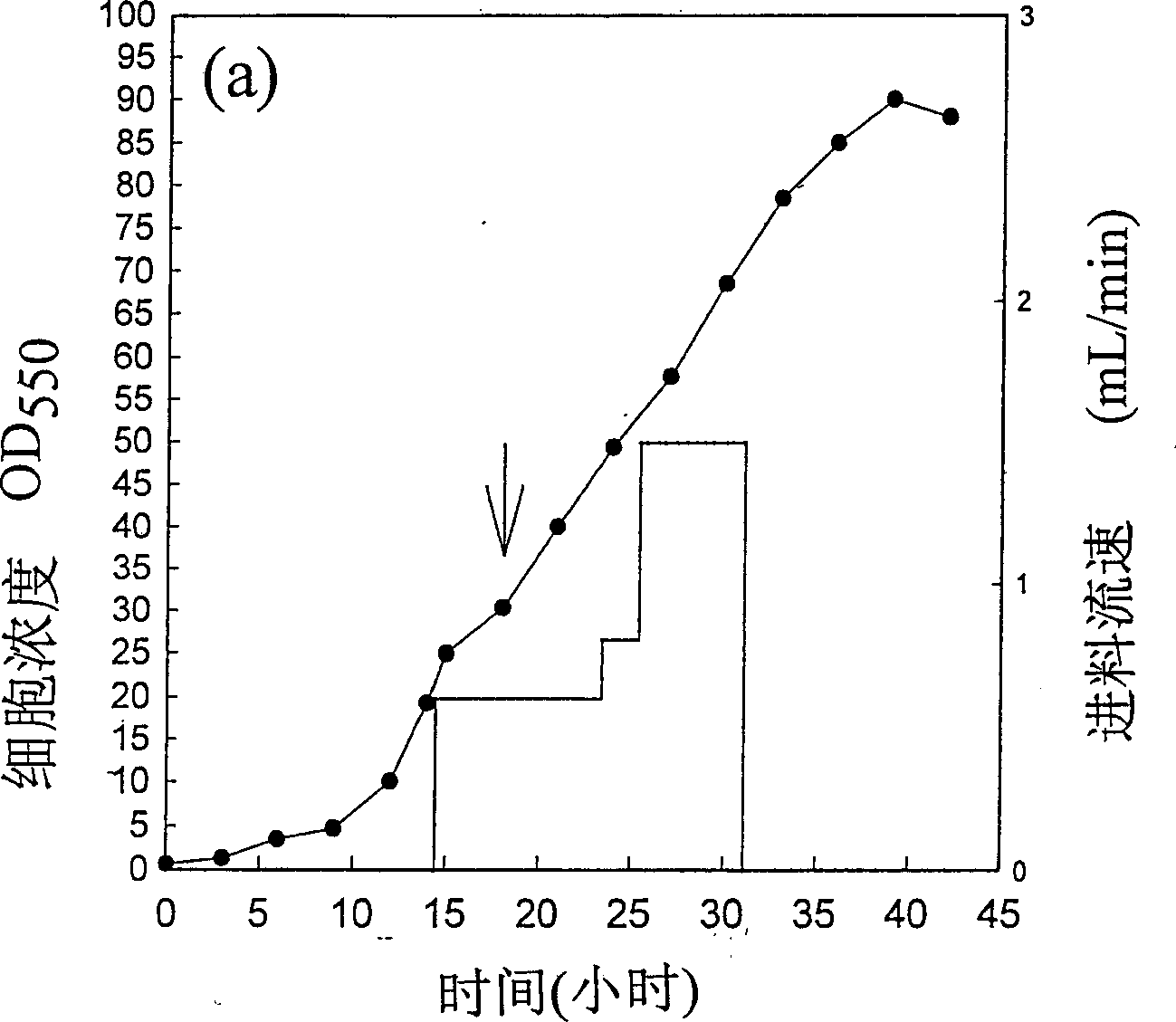
[0085] Example (3), using heat-induced control of T7 expression system to produce recombinant protein in laboratory fermenter scale.
[0086] The practicality of a gene expression system determines that it is still feasible when it is scaled up. Based on this, we use the fed batch fermentation method to test the potential practicality of the strains developed in the present invention.
[0087] A. Strain culture and nutrient matrix formula
[0088] A 5L laboratory-scale fermenter (Biostat, B. Braun, Germany) was used for the culture of scale-up strains. The operating conditions of the fermenter were set at 30° C., pH 7.0, and the dissolved oxygen was 15% of the saturated dissolved oxygen. The bacterial classification that cultivates 300mL with shaking flask earlier is as inoculum (embodiment (one) item A), and the composition of nutrient matrix is that every liter of solution contains 3g potassium phosphate, 6g disodium phosphate, 1g ammonium chloride, 0.248g Magnesium sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com