9, 9-bis(triphenyl amino) fluorine derivatives and preparation and use thereof
A triphenylamine-based and triphenylamine-based technology, which is applied in semiconductor/solid-state device manufacturing, electrical components, circuits, etc., can solve the problems that the hole transport layer has not been reported, the price of PEDOT is expensive, etc., and achieve good hole transport performance, Excellent electrochemical performance and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
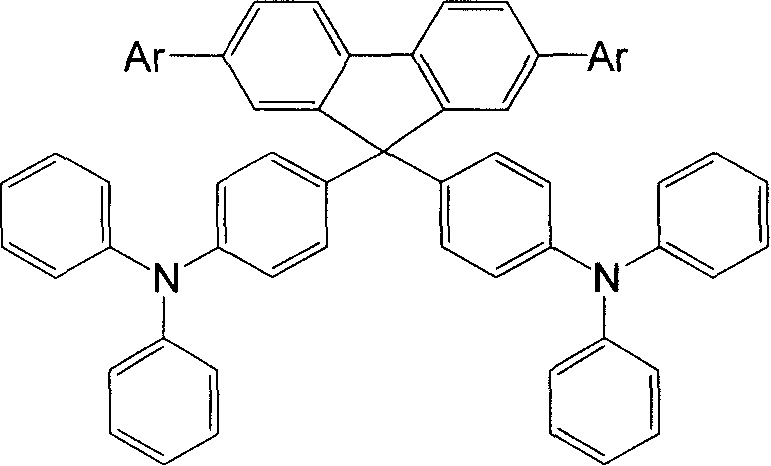
[0026] Example 1 Synthesis of 2,7,9,9-tetrakis(triphenylamino)fluorene (No. XB10)
[0027] Under the protection of argon gas, 338mg (1mmol) 2,7-dibromo-9-fluorenone, 3.43g (14mmol) triphenylamine and 91mg (1mmol) methanesulfonic acid were added to a 100mL reaction flask in sequence, and the gas in the reaction flask After replacing with an inert gas, the mixture was heated to 140°C and stirred at this temperature for about 8 hours to obtain a reddish brown solution, cooled to room temperature, dissolved in dichloromethane, washed with saturated aqueous sodium carbonate solution and dried with anhydrous sodium sulfate. After concentration, column chromatography (silica gel, petroleum ether / dichloromethane / ethyl acetate elution) separated 0.633 g of 2,7-dibromo-9,9-bis(triphenylamino)fluorene with a yield of 78.1%.
[0028] Add 3.24g (10mmol) of triphenylamine bromide to n-BuLi at -78°C. After reacting for 1 hour, add 2.82g (15mmol) of triisopropanol borate, stir for 2 hours, raise ...
Embodiment 22
[0031] Example 22 Synthesis of 7-bis(2-naphthyl)-9,9-bis(triphenylamino)fluorene
[0032] The preparation method of 2,7-dibromo-9,9-bis(triphenylamino)fluorene is the same as in Example 1.
[0033] Under the protection of argon gas, 10.35g (50mmol) 2-bromonaphthalene, 1.32g (55mmol) magnesium chips and 100mL tetrahydrofuran were sequentially added to the reaction flask. After the reaction was refluxed for 4 hours, it was cooled to -78℃, and 14.1g (75mmol) ) Triisopropanol borate, stir for 2 hours, raise the temperature to room temperature, and continue stirring for 10 hours to obtain a green solution. Add 500 mL of 2N hydrochloric acid solution, stir at room temperature for 2 hours, and then add water. After extraction with ether, washing with saturated brine, drying with anhydrous sodium sulfate and concentration, toluene was recrystallized to obtain 3.18 g of 2-naphthyl monoborate with a yield of 37%.
[0034] Under the protection of argon, in a 100mL reaction flask, sequentiall...
Embodiment 3 3
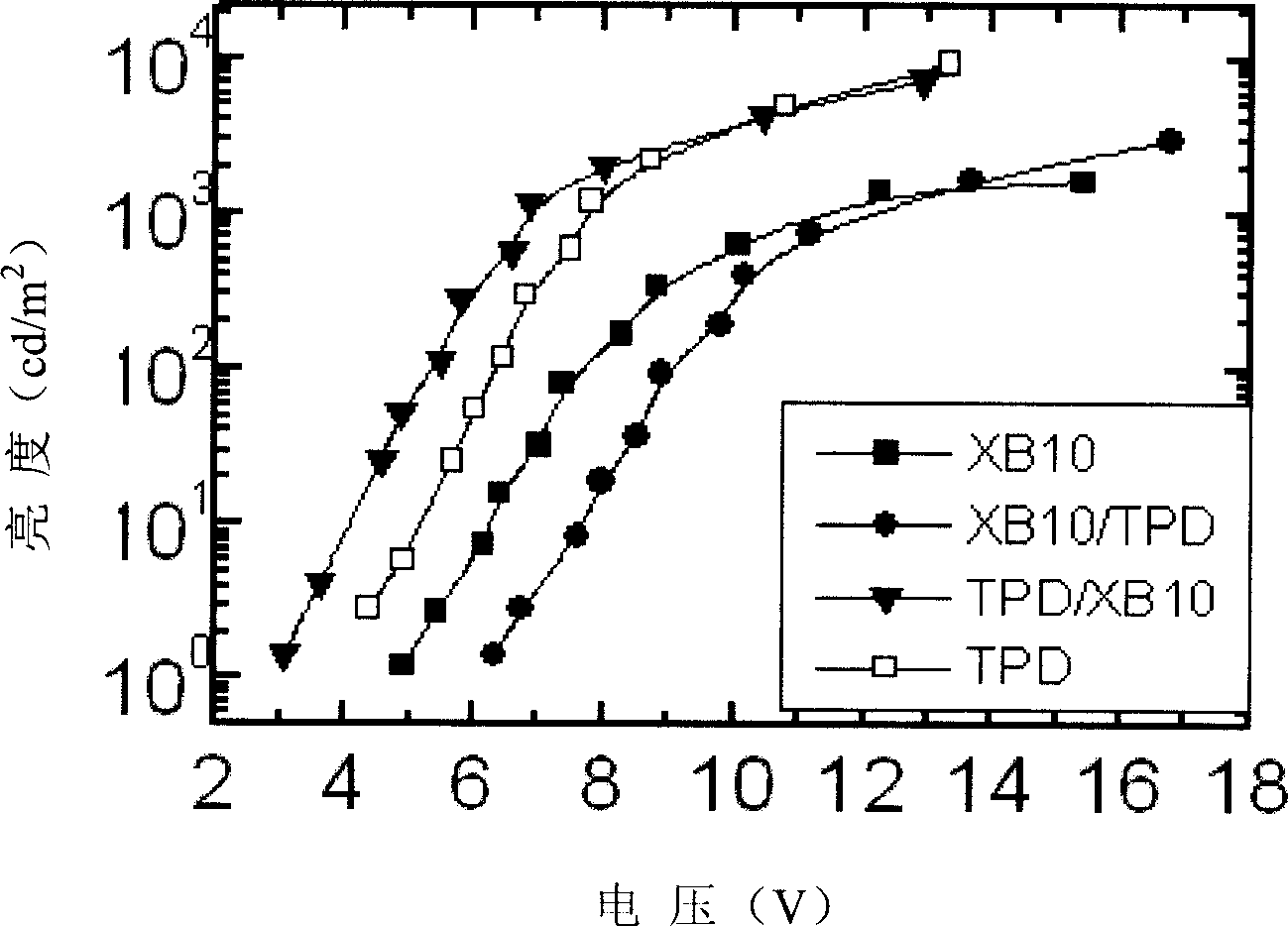
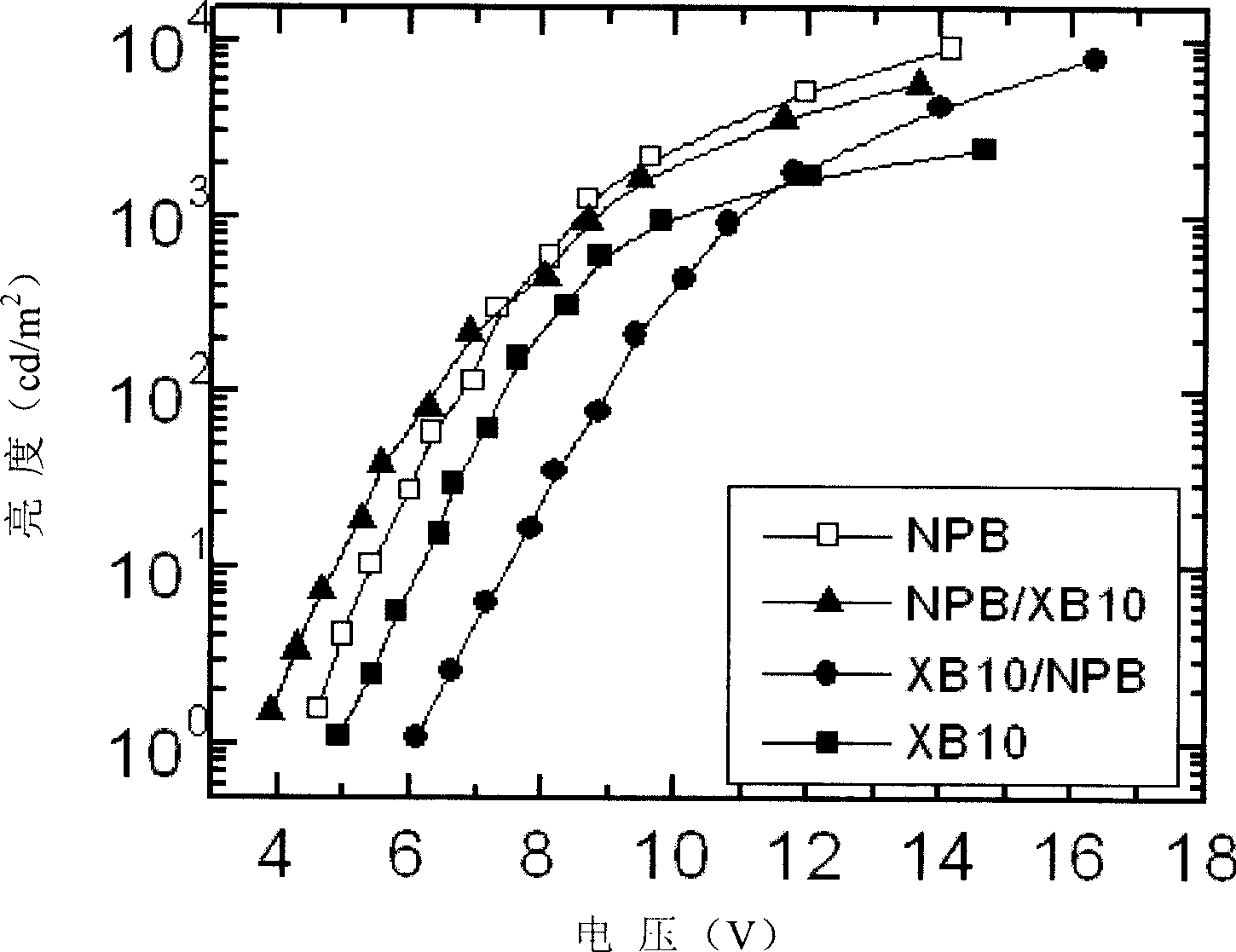
[0036] Example 3 Fabrication and performance of light-emitting devices when triphenylamine-fluorene compound is used as the hole-transporting layer of TPD
[0037] Use TPD as the hole transport layer, the triphenylamine-fluorene compound (No. XB10) obtained in the foregoing Example 1 as the hole transport layer, Alq 3 It is the light-emitting layer and the electron transport layer, using ITO glass as the anode, aluminum fluoride as the electron injection layer, and aluminum as the cathode. The light-emitting device is prepared by vacuum evaporation. See the voltage and brightness curve of the device figure 1 .
[0038] The symbols in the figure are:
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com