Near infrared meso-position nitrogen and sulfur substituted hepta-methyl-cyanine fluorochrome for bioanalysis
A technology of near-infrared and cyanine dyes, applied in the field of fluorescent dyes, which can solve the problems of decreased light stability, complex dye synthesis, separation and purification, small Stokes shift, etc., and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
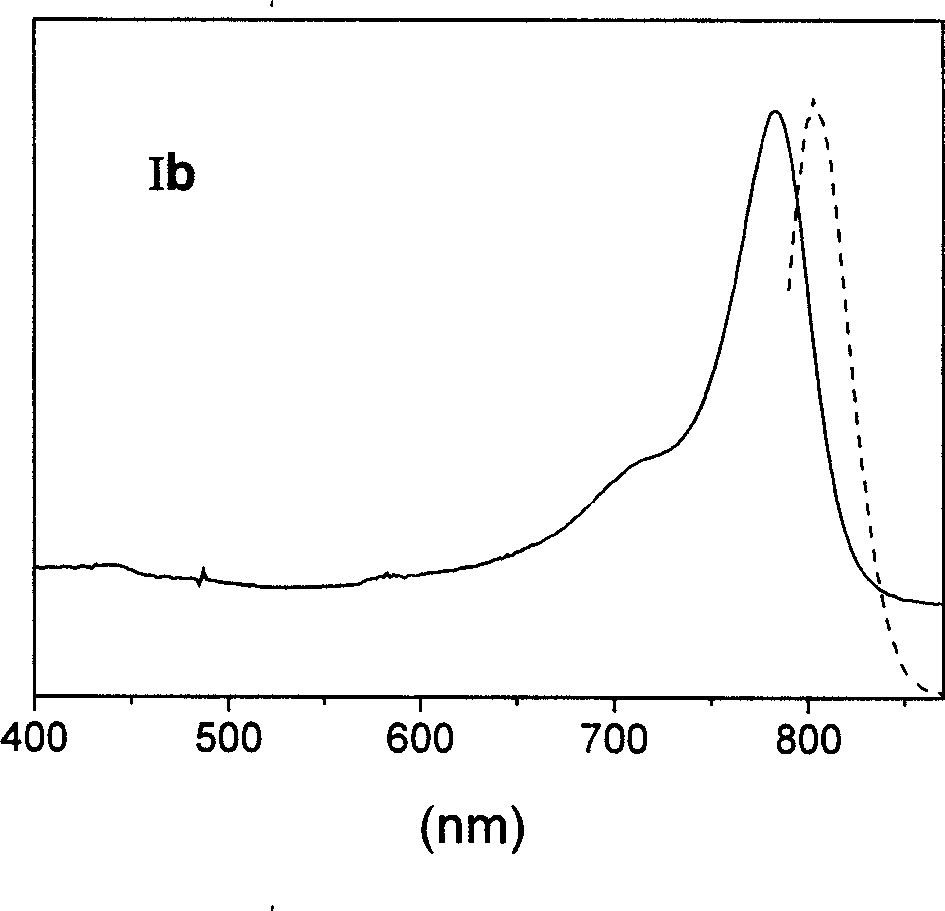
[0058] Synthetic route of parent dye Ib containing chlorine bridged ring
[0059]
[0060] (1) Synthesis of intermediate 2,3,3-trimethyl-3H-indoline-5-potassium sulfonate
[0061] In a 1000ml three-neck flask, add 150ml of glacial acetic acid, 84ml (0.8mol) of 3-methyl-2-butanone and 50g (0.26mol) of p-hydrazinobenzenesulfonic acid in sequence. The mixture was heated to reflux for 3 hours, the reactant was poured into a beaker, and after standing to cool, a pink solid precipitated out. Filtration and vacuum drying gave 48 grams of 2,3,3-trimethyl-3H-indoline-5-sulfonate, 75% yield, R1=0.58, and the developer was n-butanol: acetic acid: water ( 2:1:5).
[0062] In a 250ml round bottom flask, add 50ml methanol, 50ml isopropanol, 48g (0.2mol) 2,3,3-trimethyl-3H-indoline-5-sulfonate and 12.3 grams (0.22mol) KOH successively . The mixture was heated to reflux, the pink color quickly receded, and a large amount of yellow solid precipitated out upon cooling. Filtration and va...
Embodiment 2
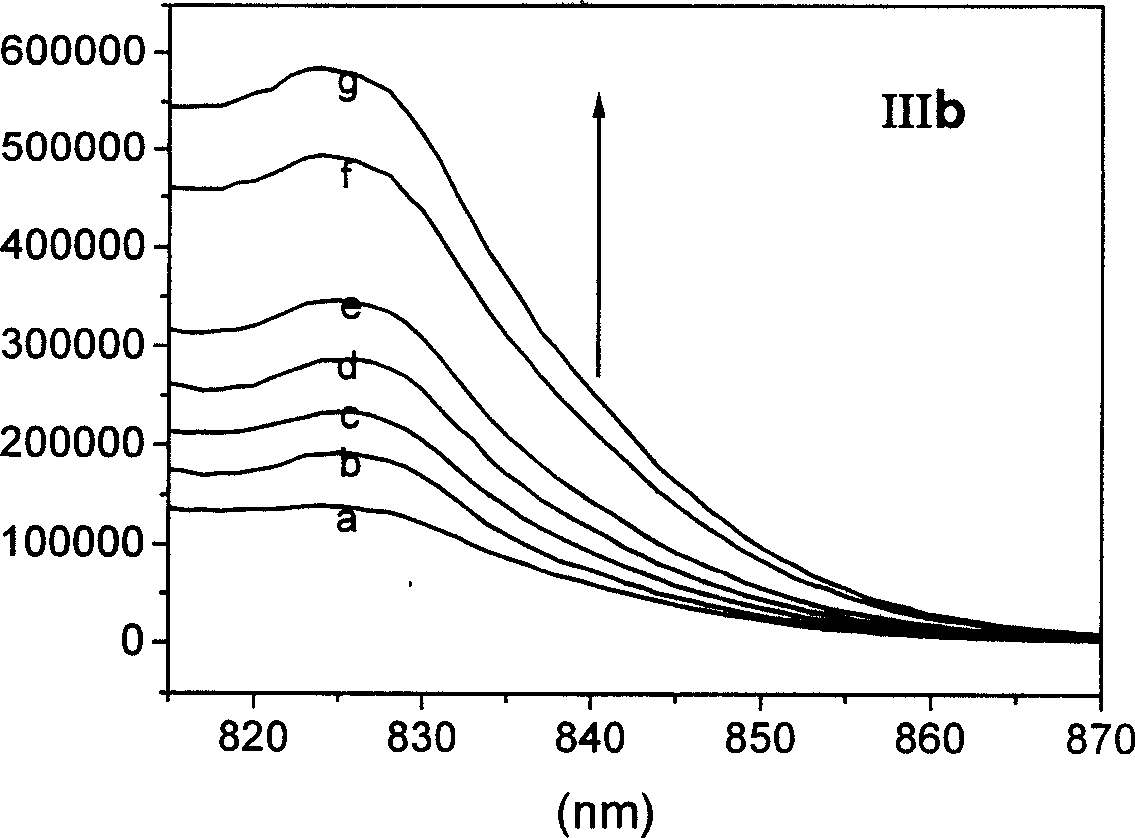
[0070] Synthesis and characterization of target dye IIa:
[0071]
[0072] The parent dye Ib was vacuum-dried at 50-60°C for 8 hours in advance, and the nucleophile aniline was purified by vacuum distillation in advance. The glassware used was pre-dried at 120°C for 3 hours, and the 50mL three-necked round-bottomed flask was cooled under a nitrogen atmosphere, and 100mg (0.12mmol) of the raw material dye Ib and 1.2mmol of the nucleophile and a magnetic stirrer were added. Under nitrogen atmosphere, about 15 mL of anhydrous DMF was added by syringe. The reaction mixture was kept at 100° C. and stirred for 2 hours under the protection of nitrogen. The reaction solution was poured into rapidly stirred 300mL ether, and a large amount of green solid precipitated. Filter the filtrate with a sand-cored funnel, dissolve the solid from the funnel with methanol, evaporate the methanol to dryness, and purify the crude dye with a reverse-phase packed column.
[0073] Dye IIa, 1 H N...
Embodiment 3
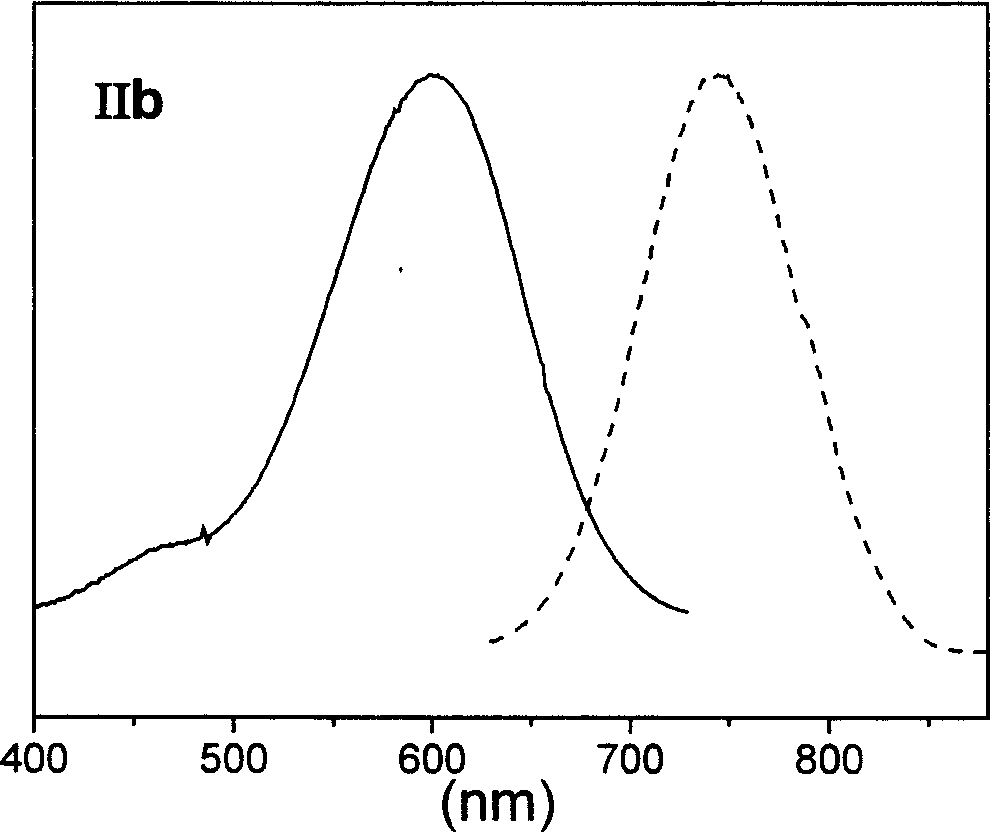
[0075] Synthesis and characterization of target dye IIb:
[0076]
[0077] The synthesis method is the same as in Example 2, wherein the nucleophilic reagent uses γ-aminobutyric acid, and the reaction temperature is 40°C.
[0078] Dye IIb, 1 H NMR (400MHz, DMSO-d6): δ0.86 (m, 2H, CH 2 ), 1.25 (m, 4H, CH 2 ), 1.63 (s, 12H, CH 3 ), 1.93 (m, 2H, CH 2 ), 2.29(t, 4H, CH 2 ), 5.20 (s, 4H, CH 2 ), 5.72(d, 2HJ=13.2Hz, CH), 7.00-7.02(d, 2H, CH), 7.25(m, 6H, CH), 7.34(m, 4H, CH), 7.53(m, 4H, CH ), 7.64 (s, 2H, CH). MS, m / z: 861.3 (M-).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com