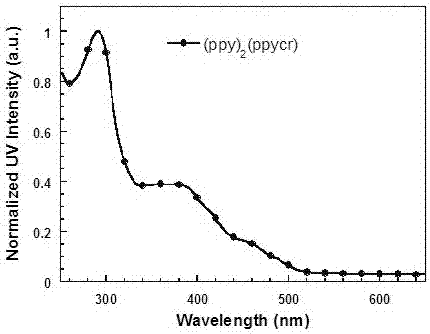
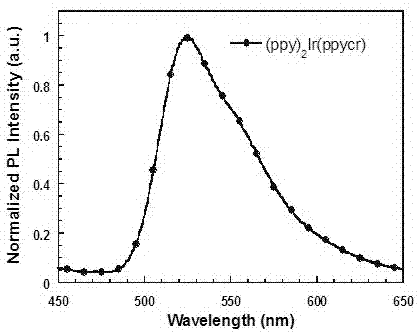
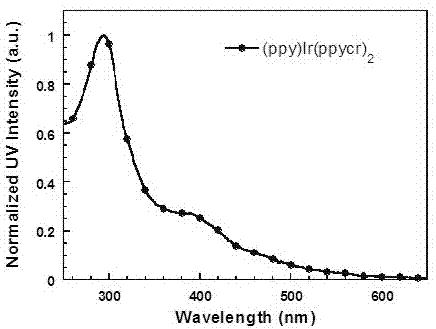
Dibenzo-18-crown-6 base cyclometal iridium complex and its application
A technology of iridium complexes and metal iridium, which is applied to compounds containing elements of group 8/9/10/18 of the periodic table, indium organic compounds, platinum group organic compounds, etc., can solve the problem of reducing the efficiency of phosphorescent devices and reducing device luminescence Efficiency and life, concentration quenching and other issues, to avoid quenching effect, improve luminous performance, improve the effect of luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of Dibenzo-18-Crown-6 Borate
[0049]
[0050] (1) Synthesis of dibenzo-18-crown-6
[0051] Put 22 g (0.2 mol) catechol, 30.03 g (0.21 mol) 1-chloro-2-(2-chloroethoxy) ethane and 110.4 g potassium carbonate in a 500 mL round bottom flask, add 350 mL of N,N-dimethylformamide (DMF) solution, the reaction was heated to reflux, and the reaction was stirred for 24 h. Cool to room temperature, pour into a large amount of water, and extract with dichloromethane 3 times. The organic layer was washed with water, dried over anhydrous magnesium sulfate, and the organic solvent was distilled off. Purified by column chromatography (silica gel as the stationary phase, petroleum ether: ethyl acetate = 4:1 as the eluent) to obtain 7.57 g of a white solid with a yield of 21%. 1 H NMR (300 MHz, CDCl 3 , TMS) δ (ppm): 6.88 (s, 8H),4.16~4.13 (t, J = 4.36 Hz, 8H), 3.93~3.90 (t, J = 4.35 Hz, 8H).
[0052] (2) Synthesis of 4-bromo-dibenzo-18-crown-6
[0053] Add 3.6 g (...
Embodiment 2
[0057] Bromination fac -Synthesis of three (2-phenylpyridine) iridium
[0058]
[0059] (1) fac -Three (2-phenylpyridine) iridium (Ⅲ) [ fac -Ir(ppy) 3 ]Synthesis
[0060] Add 1.5 g (10 mmol) of 2-phenylpyridine, 21 mL of ethylene glycol monoethyl ether and 7 mL of water into a 50 mL three-neck flask, and quickly add 1.4 g (4.0 mmol) of IrCl under argon protection 3 •3H 2 O, 100 °C constant temperature reaction for 20 h. After cooling, a yellow solid was produced, which was filtered by suction, washed with water and a little ethanol successively, and dried in vacuum to obtain 2.42 g of a yellow powder. The product was directly used in the next reaction without further separation and purification.
[0061] In a 50 mL three-neck flask, add 0.8 g (0.75 mmoL) of the reaction product of the previous step, 0.62 g (4.0 mmoL) of 2-phenylpyridine, 424 mg (4.0 mmoL) of sodium carbonate, 0.6 mL of acetylacetone, and 0.6 mL of three Ethylamine and ethylene glycol 30 mL. Under t...
Embodiment 7
[0069] Dibenzo-18-crown-6 radicalization fac -Synthesis of three (2-phenylpyridine) iridium
[0070]
[0071] (1) Two (2-phenylpyridine)-(2-(5-(18-crown-6-yl)phenyl)pyridine) iridium (Ⅲ) [(ppy) 2 Synthesis of Ir(ppycr)]
[0072] 368 mg (0.5 mmol) of bis(2-phenylpyridine)-(2-(5-bromophenyl)pyridine)iridium(Ⅲ)[(ppy) 2 Ir(ppyBr)], 292 mg (0.6 mmol) of dibenzo-18-crown-6 borate and 20 mL of 2.0 M potassium carbonate aqueous solution, as well as 40 mL of toluene and 20 mL of ethanol were added to a 150 mL two-necked flask, argon Add 30 mg (0.025 mmol) tetrakis(triphenylphosphine) palladium under protection, heat to 90 °C, and react for 24 h. After cooling to room temperature, the reaction solution was poured into deionized water, extracted three times with dichloromethane, and the organic phases were combined and washed with water. Anhydrous MgSO 4 After drying overnight, the volatile solvent was evaporated by a rotary evaporator. The crude product was separated and purifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com