Wet treatment method of iron containing nickel sulfide material
A technology of wet treatment and nickel sulfide, applied in the direction of improving process efficiency, etc., can solve the problems of high production cost, environmental pollution, large nickel loss, etc., to simplify the process and equipment, reduce reagent consumption, and reduce iron slag The effect of slag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
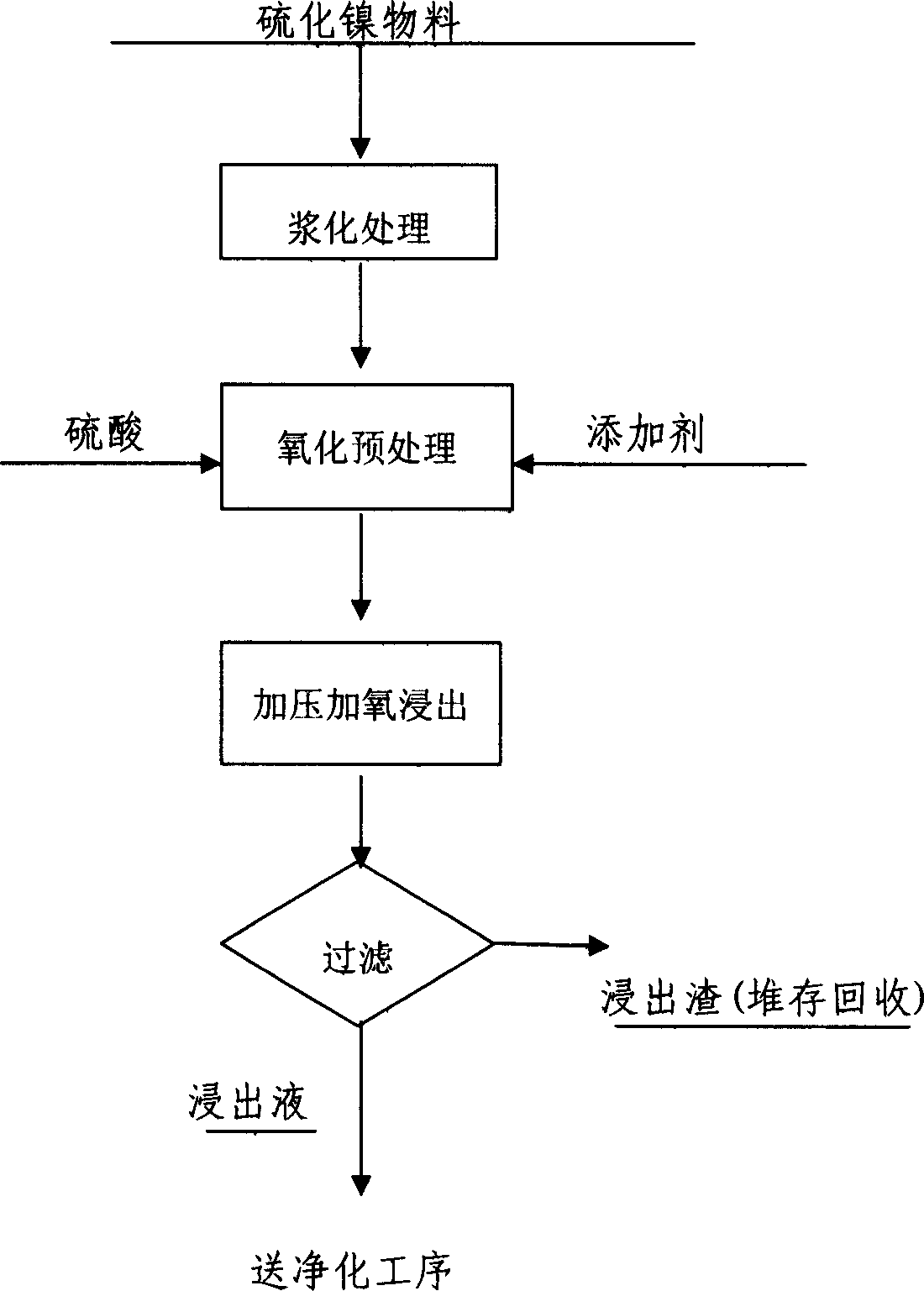
[0020] In a 50-liter stainless steel pressurized reactor, put in 24 liters of water and 10 kg of wet nickel sulfide. The main components (mass%, dry basis) are: Ni 10.27, Fe 27.11, Mn 0.016, Mg 1.06, S 29.95, Ca2 O 46.18. Heat up to 90°C, feed industrial oxygen, control oxygen pressure 0.3MPa, after 40 minutes, add 450 grams of ammonium sulfate, 85 milliliters of industrial sulfuric acid (93%), 10 grams of lignocalcium, heat up to 145°C, feed industrial oxygen, Control the reaction pressure to 1.0 MPa, cool, filter and wash after 5 hours, and produce 31 liters of leachate (including washing water), and its composition is (g / L): Ni 17.41, Fe14.11, Mg 1.56, Co 0.983. The output leaching residue was 5.3 kg (dry basis), containing 0.246% Ni. Add 5.3 kg of sodium sulfate to the leaching solution in a 50-liter stirring tank, heat it to 85-90°C under normal pressure, and add industrial-grade calcium carbonate powder. The process controls the pH value to 1.5-2.0. After stirring for 2 ...
Embodiment 2
[0022] The above iron removal purification solution is heated to 90°C under normal pressure in a 50-liter stirring tank, and 120 grams of sodium fluoride is added with stirring, and neutralized with industrial grade calcium carbonate powder to a pH value of 5.5. Cobalt is extracted after kerosene is produced into nickel soap. The raffinate is 27.5 liters, and its composition is (g / L): Ni20.44, Co 0.011; the stripping liquid is 3 liters, and its composition is (g / L): Co 8.79, Ni0.599; add sodium carbonate to the raffinate to neutralize to pH 7.5, filter and wash the filter cake, add sulfuric acid to dissolve, concentrate and crystallize to obtain 2.05 kg of nickel sulfate, the composition is (g / L): Ni21.67, Co 0.005, Fe 0.0017, Cu 0.0021, Zn 0.0045, Pb 0.0016, Ca 0.0018, Mg 0.0045.
[0023] The technical and economic indicators obtained in the above examples are as follows:
[0024] The leaching rate of nickel is 97.64%, the leaching rate of iron is 29.98%, the impurities of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com