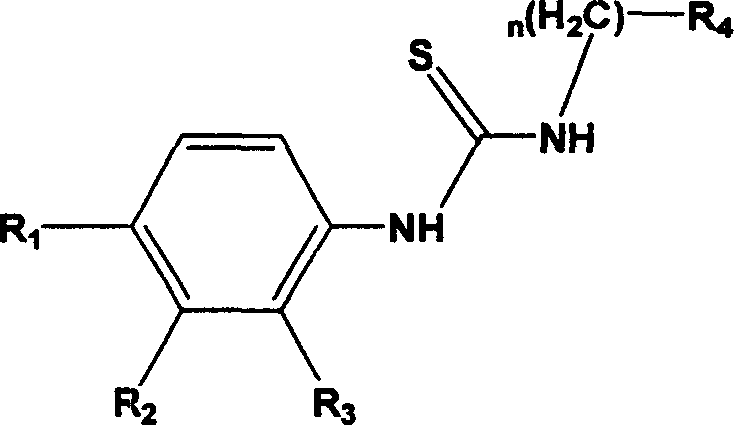
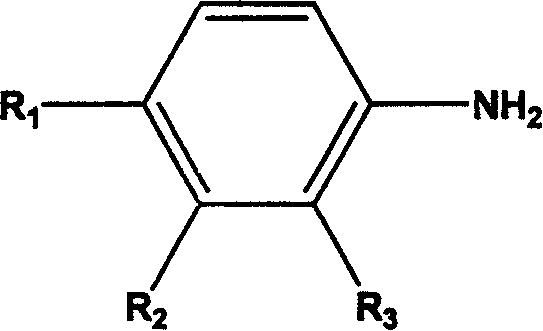
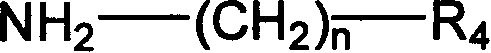Thiourea kind compund with inhibiting virus capsid protain activity and its preparation process and application thereof
A compound, sulfur-based technology, applied in organic active ingredients, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve problems such as drug resistance, treatment measures, and adverse effects of disease outcomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation of 3-chloro-4-methyl-phenylisothiocyanate
[0066] Cool in an ice-salt bath, under mechanical stirring, add 3-chloro-4-methylaniline dissolved in an appropriate amount of methanol dropwise into 4.3ml of carbon disulfide and 9.0ml of ammonia water, stir for about 1 hour, then let stand for 2 hours (no ice-salt bath). Add appropriate amount of water and heat slightly to dissolve it. Pour this solution into a saturated copper sulfate solution of 24 g of copper sulfate pentahydrate, and stir for a certain period of time. The upper product was suction filtered, and the filter residue was dried naturally.
[0067] Add an appropriate amount of toluene to the dried filter residue, stir mechanically for 3 hours, remove insoluble matter by filtration, and evaporate the filtrate to remove the solvent under reduced pressure. Distilled under reduced pressure to obtain 5.21 g of 3-chloro-4-methyl-phenylisothiocyanate as a pale yellow liquid.
Embodiment 2
[0068] Example 2 Preparation of 2-[(dimethylamino)-methyl]-5-hydroxymethyl-furan
[0069] Put 20 g of furfuryl alcohol, 19.8 g of dimethylamine hydrochloride, and 4.6 g of paraformaldehyde in a reaction vessel, heat to reflux for 1 hour with electromagnetic stirring, add 3.1 g of paraformaldehyde, heat to reflux for 1 hour, and evaporate the solvent under reduced pressure. Adjust to strong alkalinity with concentrated sodium hydroxide solution (0.1g / ml), extract with 100ml of anhydrous ether three times, and dry the extract with anhydrous sodium sulfate. The solvent was evaporated from the extract under reduced pressure, and 16.7 g of 2-[(dimethylamino)-methyl]-5-hydroxymethyl-furan was obtained by distillation under reduced pressure.
Embodiment 3
[0070] Example 3 Preparation of 2-[({5-[(dimethylamino)-methyl]-2-furan}-methyl)-thio]-ethylamine
[0071] Under cooling and stirring in an ice bath, add 16.7 g of 2-[(dimethylamino)-methyl]-5-hydroxymethyl-furan obtained in Example 2 dropwise into 12 g of cysteamine hydrochloride and 40 ml of concentrated hydrochloric acid at 0°C Reaction 18h. Sodium carbonate and sodium hydroxide powder were added to the reaction system to adjust the pH to strong alkalinity, extracted three times with 100 ml of anhydrous ether, and the extract was dried over anhydrous sodium sulfate. The solvent was evaporated from the extract under reduced pressure, and 2-[({5-[(dimethylamino)-methyl]-2-furan}-methyl)-thio]-ethylamine was obtained by distillation under reduced pressure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com