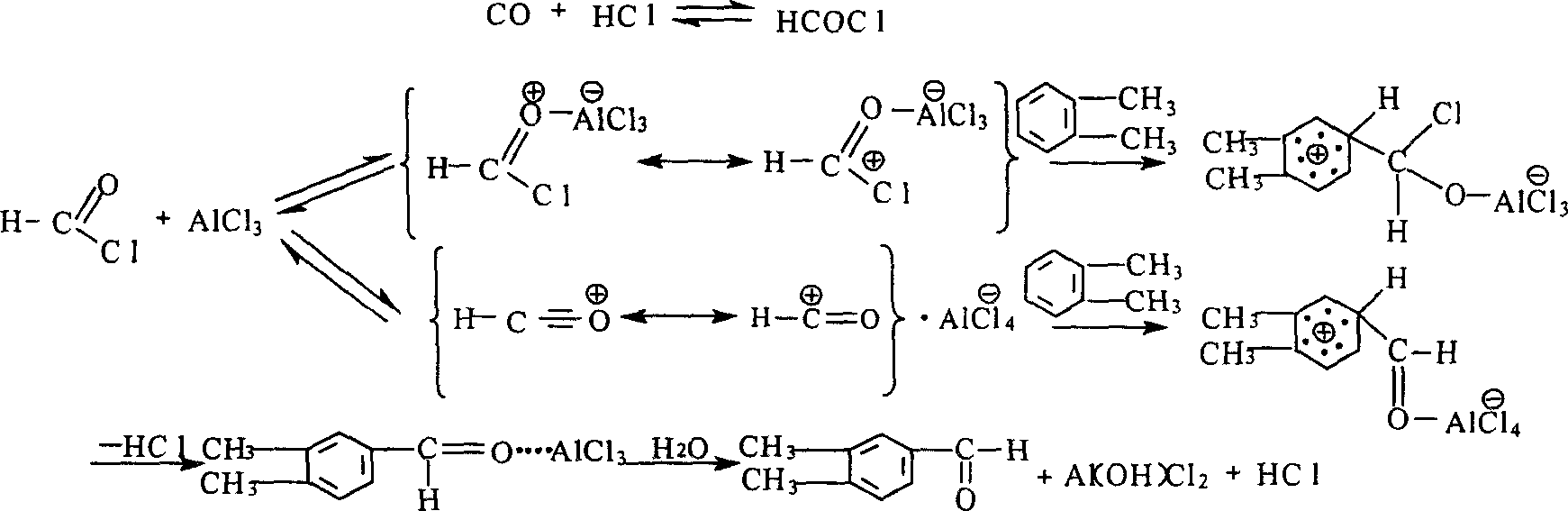High purity 3,4-dimethyl benzaldehyde preparation method
A high-purity technology of dimethylbenzaldehyde, which is applied in the direction of carbon monoxide reaction preparation and organic chemistry, can solve problems such as difficult separation, high technical difficulty, and low product purity, and achieve mild reaction conditions, high product selectivity, and catalytic Performance-specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
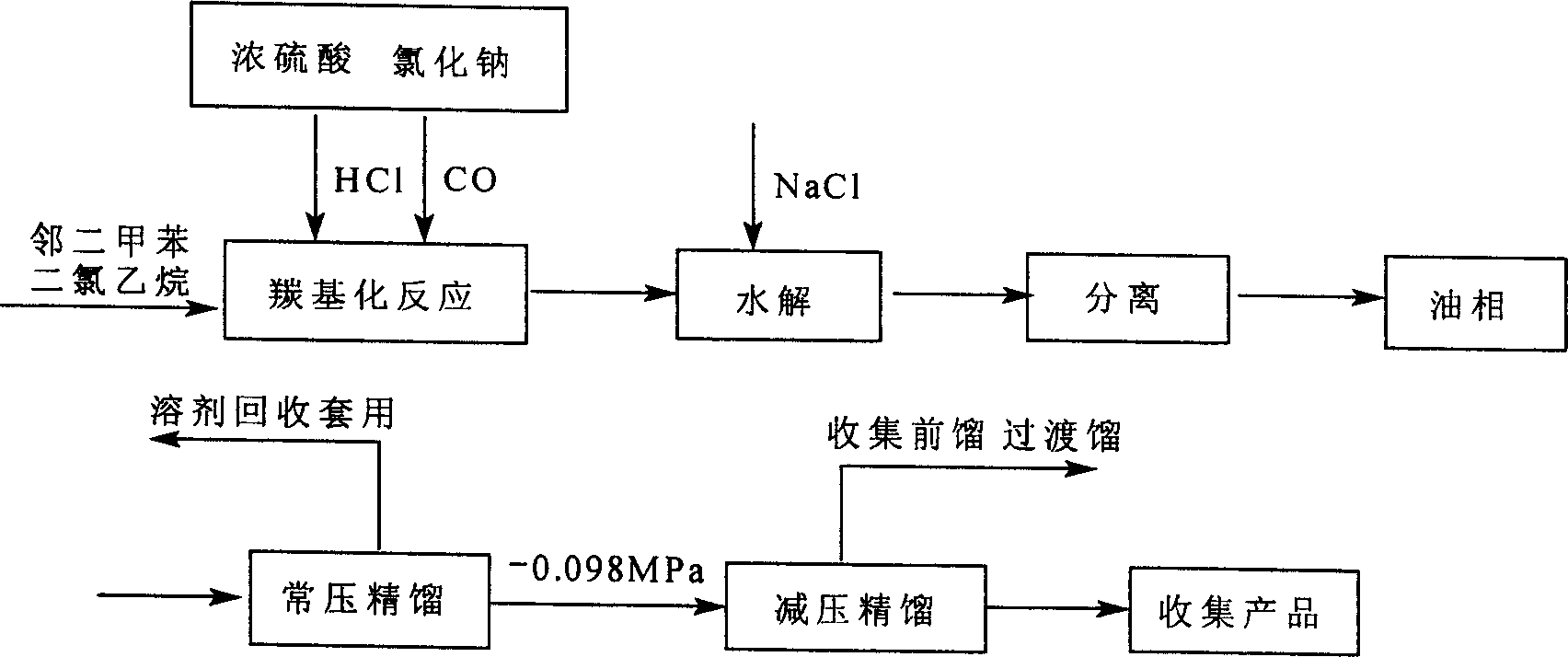
[0026] In a 1000ml four-neck flask, add weighed 216g o-xylene and 450g 1,2-dichloroethane, start the stirrer, add ice brine into the brine pot, and cool the contents of the reaction flask to Below -5℃, then add 30g cuprous chloride and 280g anhydrous aluminum trichloride in sequence, strictly control the operating temperature at -10~0℃, then open the CO cylinder valve, then open the hydrogen chloride generator concentrated sulfuric acid valve, slowly Drop concentrated sulfuric acid into the concentrated hydrochloric acid and sodium chloride bottles. At this time, there is hydrogen chloride gas overflowing from the liquid. Strictly control the volume flow ratio of CO and HCl to 1:1. The reaction takes about 8 hours, and continuous sampling and tracking at the end of the reaction Analysis shows that when the conversion rate of the reaction reaches 70%, the reaction is stopped, the CO and HCl vent valves are closed, and stirring is continued for 15 minutes, and then the above-mention...
Embodiment 2
[0028]In the same device as in Example 1, add the same amount of o-xylene and 1,2-dichloroethane as in Example 1, turn on the stirrer, add ice brine to the brine pot, and pre-load the contents of the reaction flask. Cool to below -5°C, then add 27g magnesium sulfate and 260g ferric chloride in sequence, strictly control the operating temperature at -10~0°C, then open the CO cylinder valve, then open the concentrated sulfuric acid valve of the hydrogen chloride generator, and add slowly Concentrated sulfuric acid is put into the concentrated hydrochloric acid and sodium chloride bottle, and the volume flow ratio of CO and HCl is strictly controlled at 1:1. The reaction takes about 8 hours. Continuous sampling and tracking analysis. When the single-pass conversion rate of o-xylene reaches 70% or more, Pour the above reactants slowly into a prepared 10%-20% 1500ml ice brine flask, extract, separate the lower acid water, put the upper oily substance in a rectifying flask, and then per...
Embodiment 3
[0030] In the same device as in Example 1, 216g o-xylene and 450g 1,2-dichloroethane were sequentially added, and then 30g cuprous chloride and 300g anhydrous cobalt acetate were sequentially added. The operating temperature was strictly controlled at -10~ 0℃, then open the CO cylinder valve, then open the concentrated sulfuric acid valve of the hydrogen chloride generator, slowly drop the concentrated sulfuric acid into the concentrated hydrochloric acid and sodium chloride bottles, strictly control the volume flow ratio of CO and HCl to 1:1, the reaction is about It takes 8 hours for continuous sampling and tracking analysis. When the end point is reached, slowly pour the above-mentioned reactants into a prepared 10%-20% 1500ml ice brine flask, extract, separate the lower acid water, and put the upper oily substance. Into the rectification flask, and then carry out vacuum distillation. The fractions are collected, and continuous sampling is performed for chromatographic tracking...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com