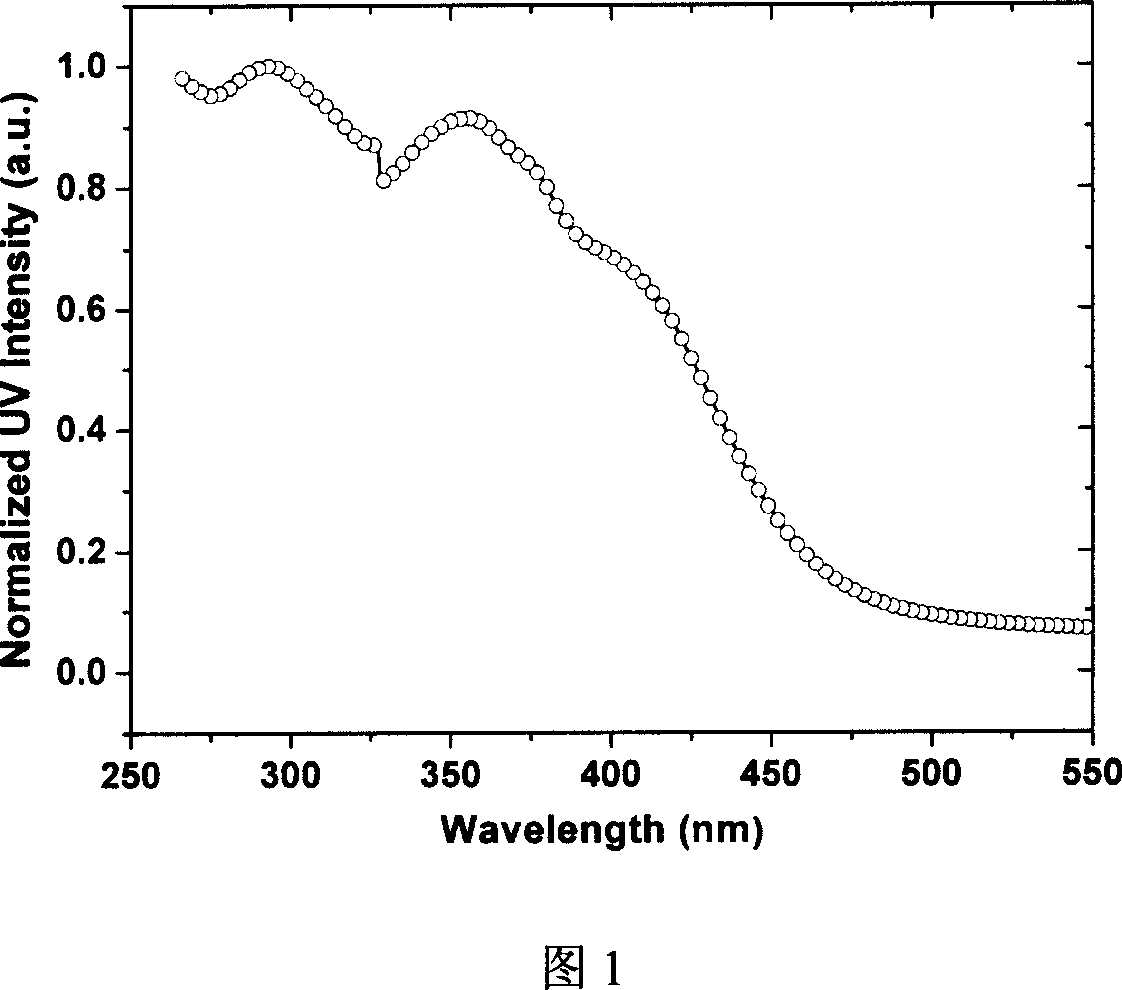
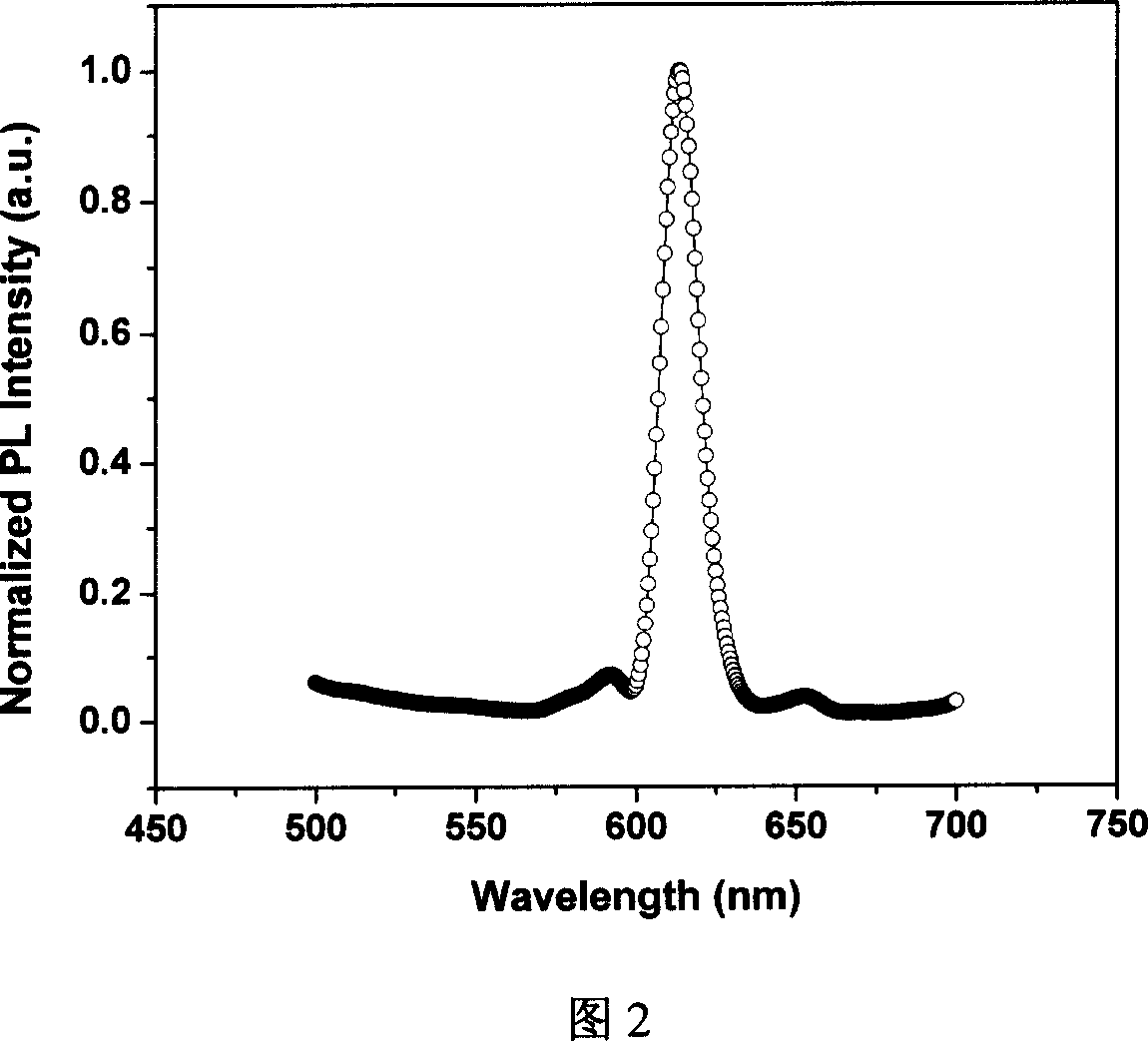
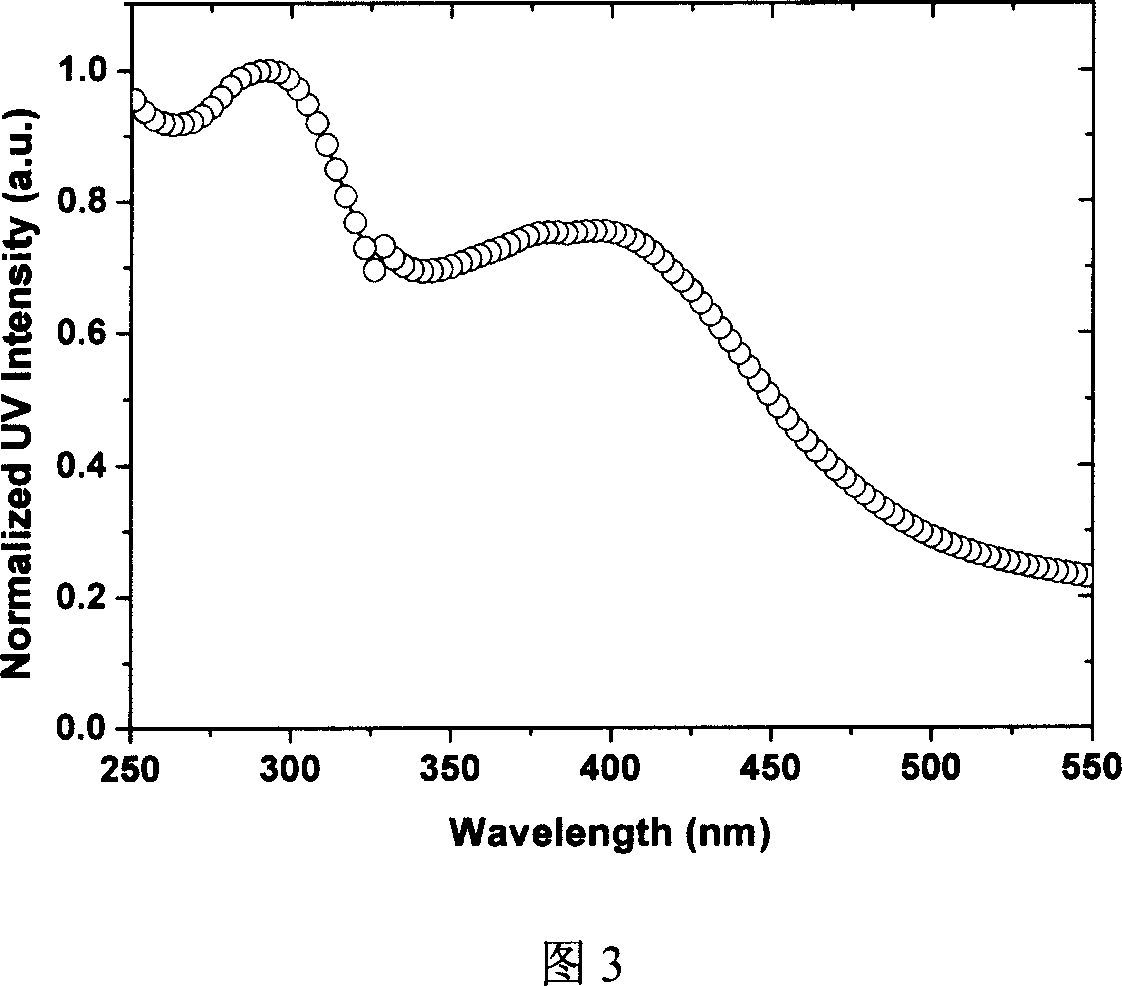
Organic eurepium compound based on functional o-phenan throline ligand and its electroluminous device
A technology of o-phenanthroline and functionalization, applied in the field of polymer electroluminescent devices, can solve the problems affecting the application of organic europium complexes, low luminous efficiency and luminous brightness, low luminous efficiency and luminous brightness, etc., to achieve improved Luminous efficiency and luminous brightness, improved electrical performance, good effect of monochromaticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthesis of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl-diphenylamine
[0052]
[0053]Add 8.87g (15.0mmol) of 4-bromotriphenylamine and 100mL of THF that was dried under sodium reflux and redistilled in a 250mL three-necked flask. 30.0mmol) of 2.2M n-butyllithium, after the dropwise addition, react at -78°C for 2h, then quickly add 10.0mL (45.0mmol) of 2-isopropoxy-4,4,5,5- Tetramethyl-1,3,2-dioxaborane, continue to react at -78°C for 3h, then naturally rise to room temperature for 24h, add an appropriate amount of distilled water to terminate the reaction, extract with anhydrous ether, wash with saturated saline , dried overnight over anhydrous magnesium sulfate, spin off the solvent, dry in vacuo, and separate by column chromatography (200-300 mesh silica gel, eluent: V 正己烷 / V 乙酸乙酯 =95 / 5), to obtain 3.8 g of white powder solid, yield 68.2%, melting point (m.p.): 92.0-93.0°C. H NMR spectrum ( 1 H NMR) (400Hz, CDCl 3 )δppm: 7.67(d, J=8.40Hz, 2H), 7.2...
Embodiment 2
[0055] Synthesis of Ligand 3,8-bis[4'-(diphenylamino)-phenyl]-1,10-phenanthroline (DTPA-Phen)
[0056]
[0057] In a 25mL three-neck flask, add 0.2523g (0.75mmol) homemade 3,8-dibromo-1,10-phenanthroline, 0.6138g (1.65mmol) 4-(4,4,5,5- Tetramethyl-1,3,2-dioxaborolan-2-yl)-phenyl-diphenylamine, 0.78g (2.5mmol) of Ba(OH) 2 8H 2 O, 6mL freshly steamed toluene, 1mL distilled water, nitrogen deoxygenation for 30min, then add 33.8mg (0.03mmol) tetrakis (triphenylphosphine) palladium, under nitrogen protection, heat to reflux for 24h, CHCl 3 Extraction, dried overnight with anhydrous magnesium sulfate, solvent removal, vacuum drying, column chromatography (200-300 mesh silica gel, eluent: V CH2Cl2 / V CH3CN =10 / 1), to obtain 548mg of light yellow solid, yield 49.8%, m.p.: 269.0~272.0°C, 1 H NMR (400Hz, CDCl 3 )δppm: 9.57(d, J=2.3Hz, 2H), 8.44(d, J=2.3Hz, 2H), 7.91(s, 2H), 7.68(d, J=8.6Hz, 4H), 7.34(t, J=7.8Hz, 8H), 7.23~7.04 (m, 16H).
Embodiment 3
[0059] Synthesis of 4-bromo-4',4"-di-tert-butyl-triphenylamine
[0060]
[0061] 11.4g (44mmol) p-tert-butyl iodobenzene, 3.4g (20mmol) p-bromoaniline, 2.0g (20mmol) anhydrous K 2 CO 3 , 0.6g (10mmol) of activated copper powder and 60mL of o-dichlorobenzene were added to a 100mL three-necked flask, heated to 180-190°C under nitrogen protection, and reacted for 21h. Cool to about 100°C, filter while hot, evaporate the solvent under reduced pressure, and separate by column chromatography (200-300 mesh silica gel, eluent: V 正己烷 / V 二氯甲烷 =1 / 10), recrystallized from methanol to obtain 2.6 g of colorless needle crystals, yield 30.5%, m.p.112-114°C. 1 H NMR (400MHz, CDCl 3 ) δppm: 7.29 (m, 2H), 7.24 (m, 2H), 7.00 (d, J=7.72Hz, 4H), 6.93 (d, J=8.2Hz, 4H), 1.31 (s, 18H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Light emitting area | aaaaa | aaaaa |
| Maximum luminescence wavelength | aaaaa | aaaaa |
| Maximum luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com