Method for separating and purifying nonane diacid
A technology for separation and purification of azelaic acid, used in chemical recovery, ozone oxidation to prepare carboxylic acid, etc., can solve the problems of complex distribution of reaction products, high equipment requirements, long separation time, etc., to facilitate large-scale continuous production and implementation Simple conditions and the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
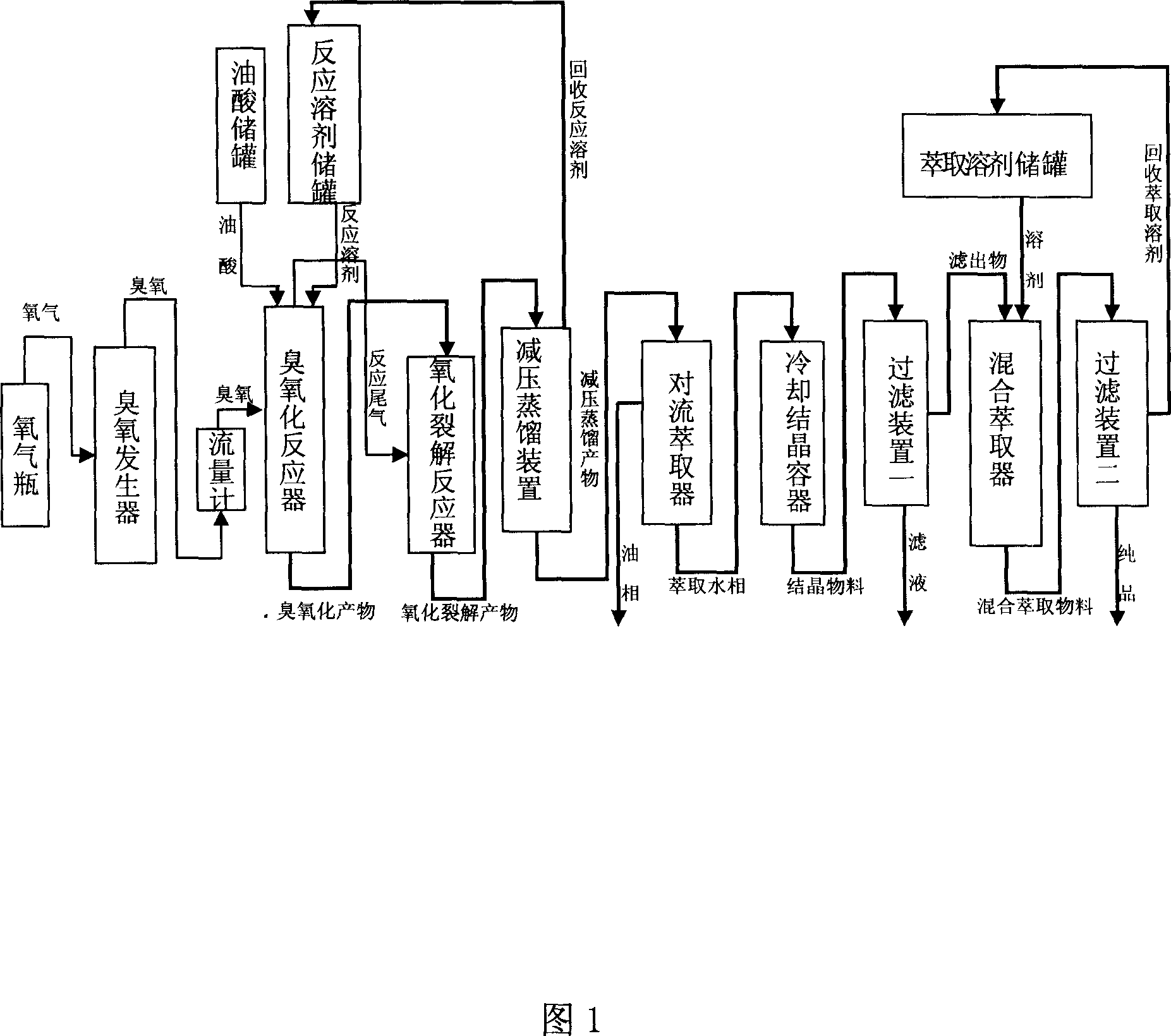
[0029] a. Stir and mix 50g oleic acid (73% by mass, 0.129mol in moles) and 200ml glacial acetic acid, and place them in a water bath at 26°C with a concentration of 108mg / L and a flow rate of 0.08m 3 / h ozone stirring reaction 2.5h;
[0030] b. Heat up, when the temperature rises to 92°C, the flow rate is changed to 0.10m 3 / h of oxygen for 2 hours of cracking reaction;
[0031] c. Recover glacial acetic acid by rotary evaporation, and then put the residue into 1000-1100ml of hot water at 90-100°C for extraction;
[0032] d, let stand to separate the liquids, put the lower water layer into a refrigerator with a constant temperature of 5°C to cool for 16h, filter, and dry to obtain 17.1g of azelaic acid crude product from the reaction solution, the yield (in terms of oleic acid) is 70.3%, and the melting point is 96- 103°C, 88% purity;
[0033] e. Weigh 10 g of the crude product and put it into 80 g of ethyl acetate, stir and mix for 3 hours at a constant temperature of 24° ...
Embodiment 2
[0035] a. Stir and mix 51g oleic acid (73% by mass, 0.132mol in moles) and 200ml glacial acetic acid, and place them in an ice-water bath at 0°C with a concentration of 101mg / L and a flow rate of 0.10m 3 / h ozone stirring reaction for 1.5h;
[0036] b. Heating up, when the temperature rises to 80°C, the flow rate is changed to 0.10m 3 / h of oxygen, reaction 1.5h;
[0037] c. Recover glacial acetic acid by rotary evaporation, and immediately put the residue into 1100-1200ml of 90-100°C hot water for extraction;
[0038] d, let stand to separate the liquids, put the lower water layer into a refrigerator with a constant temperature of 5°C to cool for 15h, filter, dry and extract 16.8g of the product azelaic acid crude product from the reaction solution, the yield (in terms of oleic acid) is 67.7%, and the melting point 95-103°C, 86% purity;
[0039]e. Weigh 10 g of the crude product and put it into 63 g of ethyl acetate, stir and mix for 4 hours at a constant temperature of 10...
Embodiment 3
[0041] a. Stir and mix 50g oleic acid (73% by mass, 0.129mol in moles) and 200ml glacial acetic acid, and place them in a water bath at 40°C with a concentration of 96mg / L and a flow rate of 0.12m 3 / h of ozone 2h;
[0042] b. Heating up, when the temperature rises to 120°C, the flow rate is changed to 0.10m 3 / h of oxygen, reaction 2.5h;
[0043] c. Rotary evaporation reclaims glacial acetic acid. Immediately put the residue into 1200ml of hot water for extraction after spinning;
[0044] d. Stand still for liquid separation, put the lower water layer into the refrigerator to cool for 16h, filter, dry and extract from the reaction solution to obtain 16.5g of azelaic acid crude product, the yield (calculated as oleic acid) is 67.8%, and the melting point is 96-105°C , with a purity of 79%;
[0045] e. Put 10 g of this crude product in 40 g of ether, stir and mix at a constant temperature of 35 ° C for 2 hours, let it stand and filter, dry and extract from the extract to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com