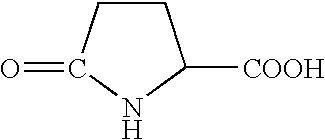
Immunoconjugates of toxins directed against malignant cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A. Example 1
Expression Pattern of RNAse in Rana pipiens Tissues
[0159] A DNA sequence corresponding to amino acid residues 16-98 of Onconase.RTM. was cloned by PCR amplification of Rana pipiens genomic DNA and sequenced. The sequence, consisting of 252 bp of DNA encoding the ribonuclease was designated Rana clone 9. Total cellular RNA was isolated from either male or female Rana pipiens tissues using RNA STAT-60 (TEL-TEST "B", Inc.) according to the manufacturer's protocol. Poly A+ containing MRNA was prepared using an Oligotex mRNA kit (Qiagen). Poly (A+) RNA was size fractionated on a 1% agarose gel containing 6% formaldehyde and blotted onto Nitran.RTM. nylon membranes (Schleicher & Schuell) in 10.times. SSC overnight. The membrane was rinsed in 2.times. SSC for 5 min, air dried and the RNA was cross linked to the membrane by exposure to UV light (Ultra-Lum) for 2 min. The RNA blot was hybridized at 42.degree. C. for 16-18 hours with a [.sup.32P]-labeled DNA probe prepared from 30...
example 2
B. Example 2
Expression of RNAse in Rana pipiens
[0166] To determine if RNAse is present in Rana pipiens oocytes or other tissues, protein extracts were isolated from various Rana pipiens tissues and separated on a 4-20% Tris-Glycine SDS--containing polyacrylamide gel. The protein extracts were transferred to a nitrocellulose membrane using 1.times.transfer buffer (Novagen) at 250 mA for 45 mm. The membrane was probed with primary and secondary antibodies as described in Chen, et al., Oncogene 12:241 (1996). The primary anti-Onconase.RTM. antibody was used at 1:100 dilution. The detecting antibody (horseradish peroxidase labeled donkey anti-rabbit Ig (Amersham)) was used at 1:2500 dilution. The antibodies were visualized using an ECL detection kit from Amersham.
[0167] The western blot analysis demonstrated that a protein of the correct size (12 kDa) was present in extracts from oocytes. Other tissues, including liver, did not contain a 12 kDa protein that reacted with the anti-Onconas...
example 3
C. Example 3
Isolation and Cloning of cDNA From Rana pipiens Liver mRNA
[0168] Liver poly (A+) RNA was purified twice using the poly (A+) Pure kit (Ambion). The cDNA library was constructed using a ZAP-cDNA synthesis kit and Gigapack II gold packaging extracts according to the manufacturer's protocol (Stratagene). The library contained about 1.5.times.10.sup.6 pfu from 5 .mu.g of liver poly (A+) RNA and was amplified once according to Stratagene's protocol. The library titer after amplification was 9.times.10.sup.9 pfu / mL. About 3.times.10.sup.5 plaques were screened by using a [.sup.32P]-labeled insert of Rana clone 9 following Stratagene's procedure. Positive clones (3alb, 4alb and 5alb) were excised from the lambda ZAP II vector and subcloned into pBluescript SF-vector. Plasmid DNA was prepared using the Qiagen spin plasmid miniprep kit.
[0169] Clone 5alb was digested with KpnI and HindIII to generate 3' and 5' protruding ends, and digested with exonuclease III to generate 5alb dele...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com