Pharmaceutical compositions with improved dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Celecoxib Sodium Salt from Aqueous Solution
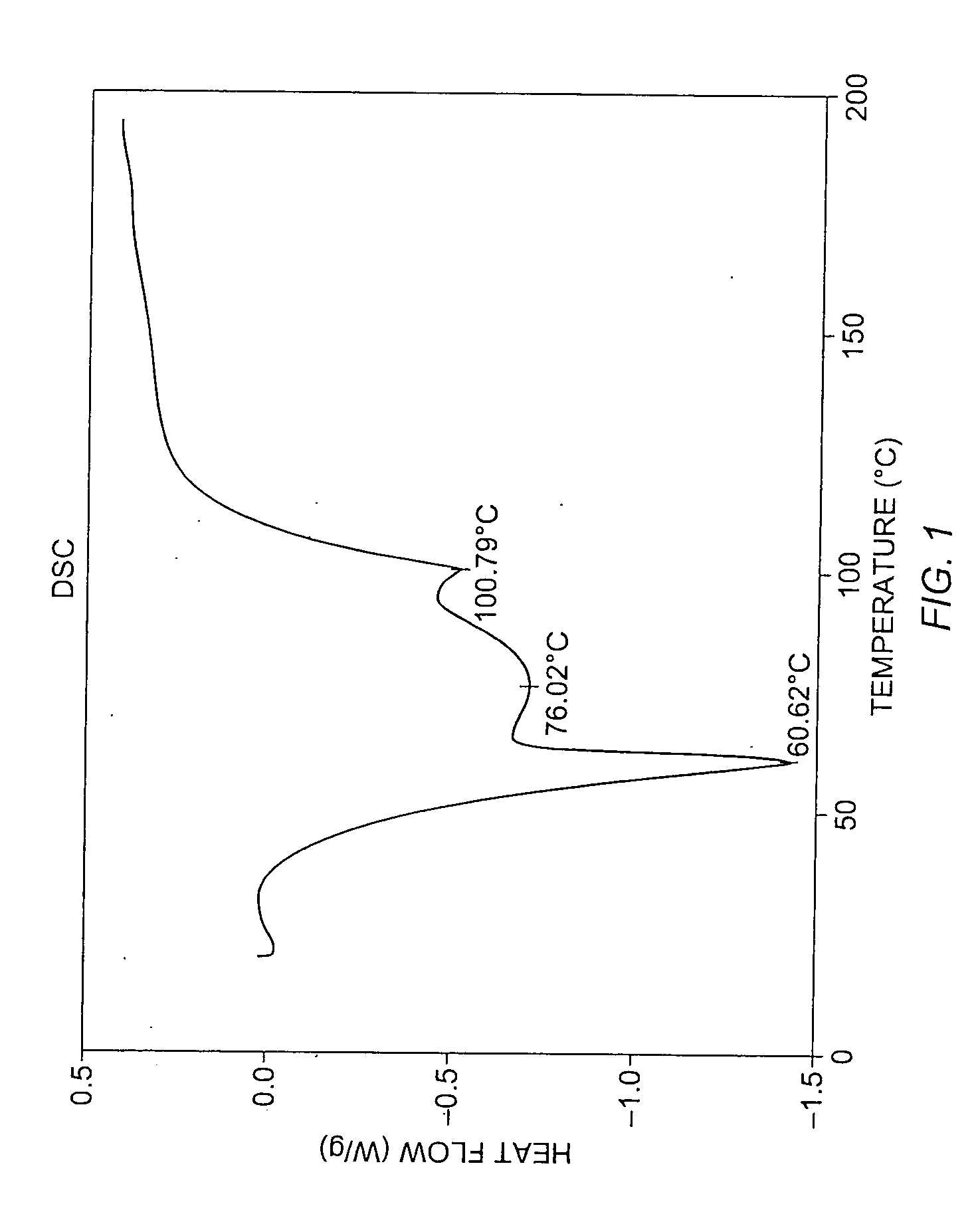
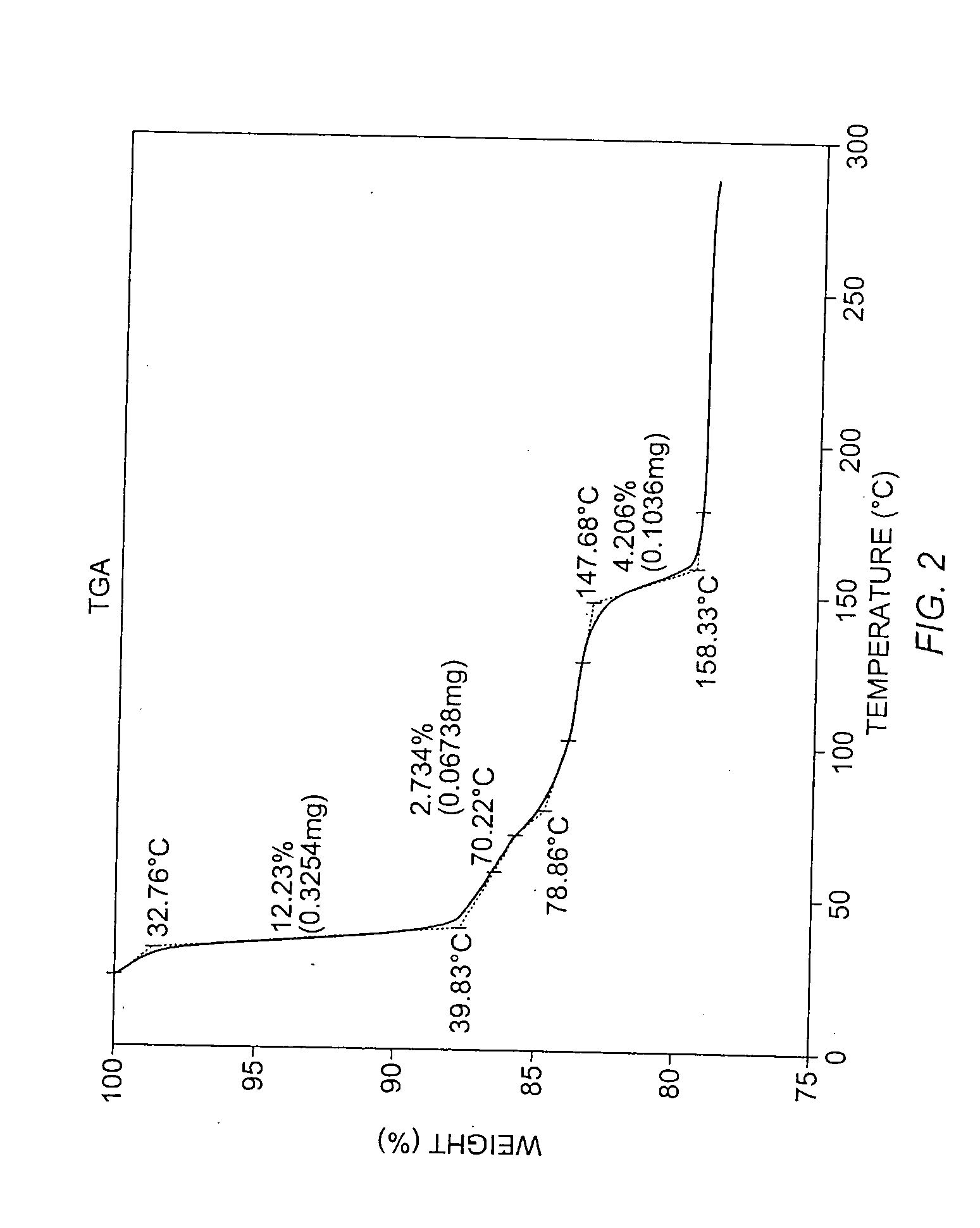
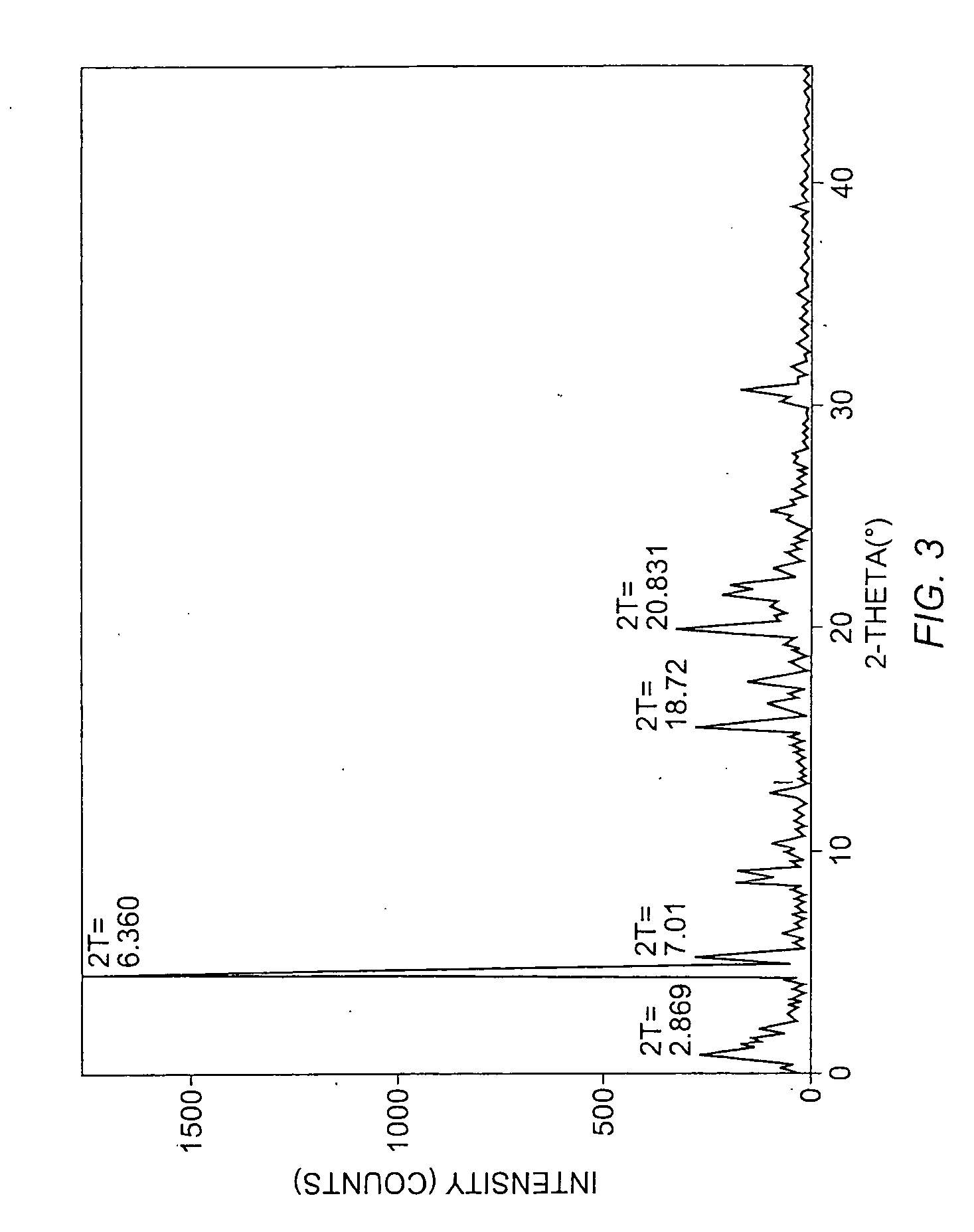
To 77.3 mg of commercially-available celecoxib was added 1.0 mL distilled water, followed by 0.220 mL of 1 M NaOH (VWR). The mixture was heated with stirring to 60° C., whereupon an additional 1.0 mL distilled water was added. Solid NaOH (22 mg) was added, and the solid NaOH and celecoxib dissolved. The mixture was heated again at 60° C. to evaporate water. About 15 mL reagent-grade ethanol was added, while the mixture was stirred and heated at 60° C. with air blowing over the solution. Heating continued until the solution was dry. The resulting material was analyzed by powder x-ray diffraction (PXRD), differential scanning calorimetry (DSC), and thermogravimetric analysis (TGA), the results of which are seen in FIGS. 1-3. The product was found to contain about 4.1 equivalents of water per equivalent of salt, although most of all of the water could be contained in the NaOH that co-precipitated with the salt.
For the DSC analysis, the pu...
example 2
Celecoxib Sodium Salt from 2-propanol Solution
To 126.3 mg of celecoxib (Fako Hazlari) was added a 1.0 mL aliquot of isopropanol, and the mixture was heated to dissolve the celecoxib. Sodium ethoxide was added as a solution 21% in ethanol (0.124 mL solution, 3.31×10−4 mol sodium ethoxide). An additional 1.0 mL of isopropanol was added. The mixture was stirred to obtain a slurry of white crystalline solids that appeared as fine birefringent needles by polarized light microscopy.
The slurry was filtered by suction filtration and rinsed with 2 mL of isopropanol. The solid was allowed to air dry before being gently ground to a powder. The product was analyzed by PXRD, DSC, and TGA as in Example 1, but a 0.5 mm capillary was used to hold the sample in the PXRD experiment. The compound lost 17.35% weight between room temperature and 120° C. The DSC trace shows a broad endothermic region, which is consistent with a loss of volatile components with increasing temperature. The endotherm p...
example 3
Celecoxib Sodium Salt from Aqueous Solution
Synthesis 1: To a vial was added 29.64 mg celecoxib and 3.00 mL of 1 N sodium hydroxide. The celecoxib dissolved immediately. After a time, the celecoxib precipitated from solution. Synthesis 2: To a vial was added 7.10 mg celecoxib and 3.00 mL of 1 N sodium hydroxide. The celecoxib dissolved. Overnight, the celecoxib precipitated and formed white, needle-like crystals. Synthesis 3: To a vial was added 17.6 mg celecoxib and 10 mL of 1 N sodium hydroxide. The celecoxib dissolved. The vial was placed in a beaker wrapped in aluminum foil and filled with a large tissue for insulation. The beaker was left and crystals formed within about 12-36 hours. Analysis: The product solids from syntheses 1 and 2 were combined and analyzed by PXRD, DSC, and TGA as in example 1, but a 0.5 mm capillary was used to hold the sample in the PXRD experiment. The product salt was found to contain about 4 equivalents of water per equivalent of salt, although a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com