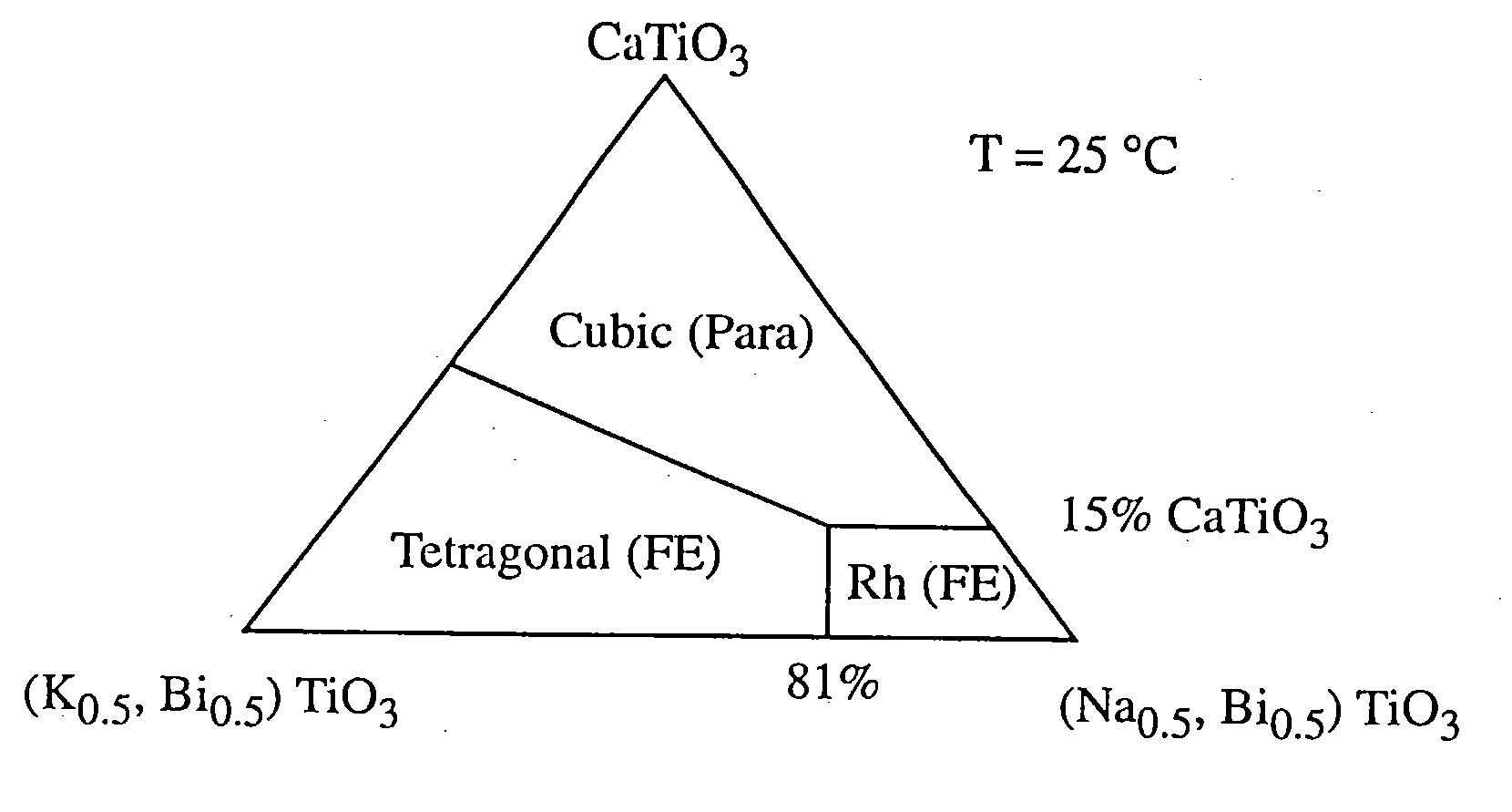
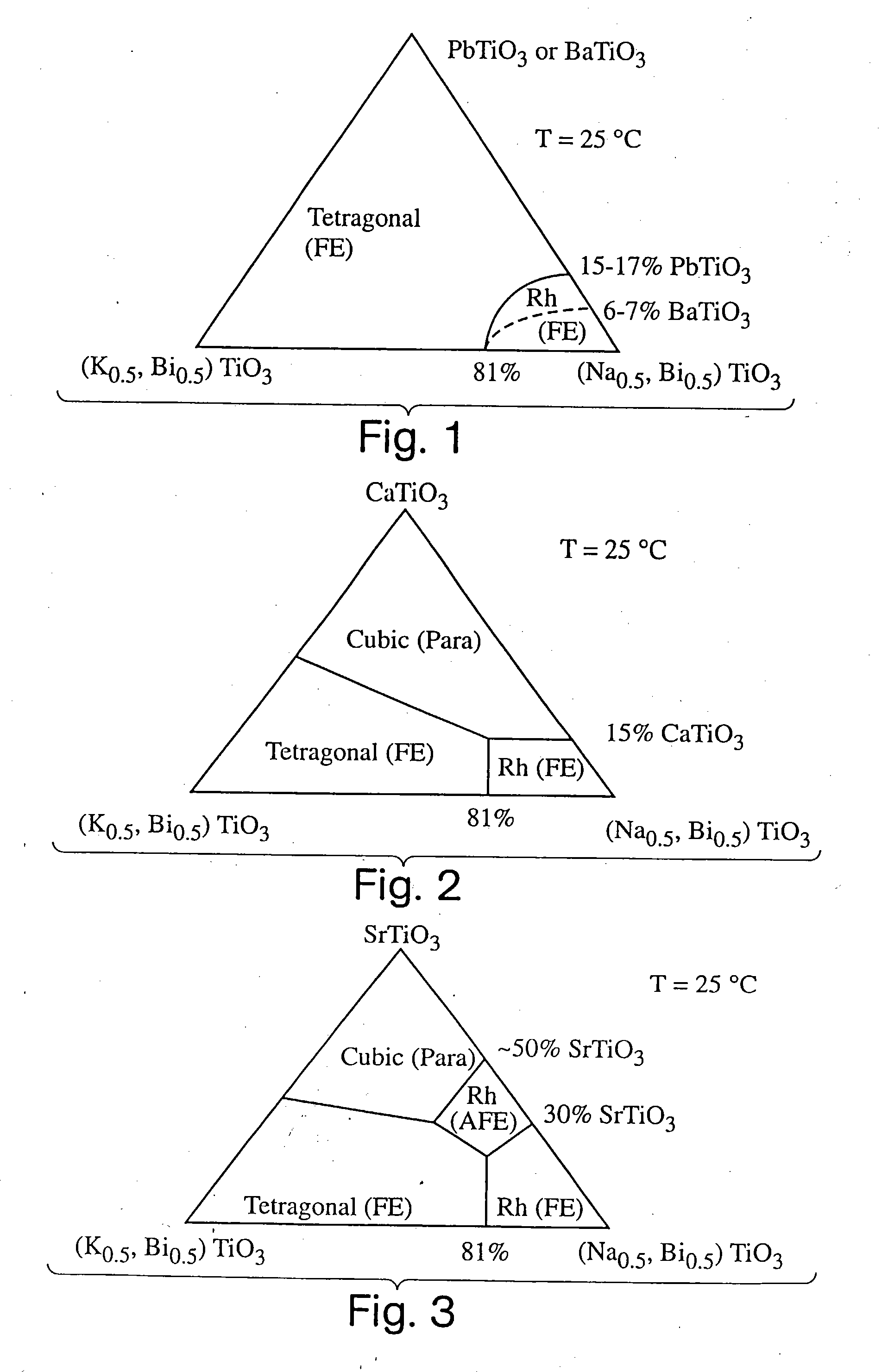
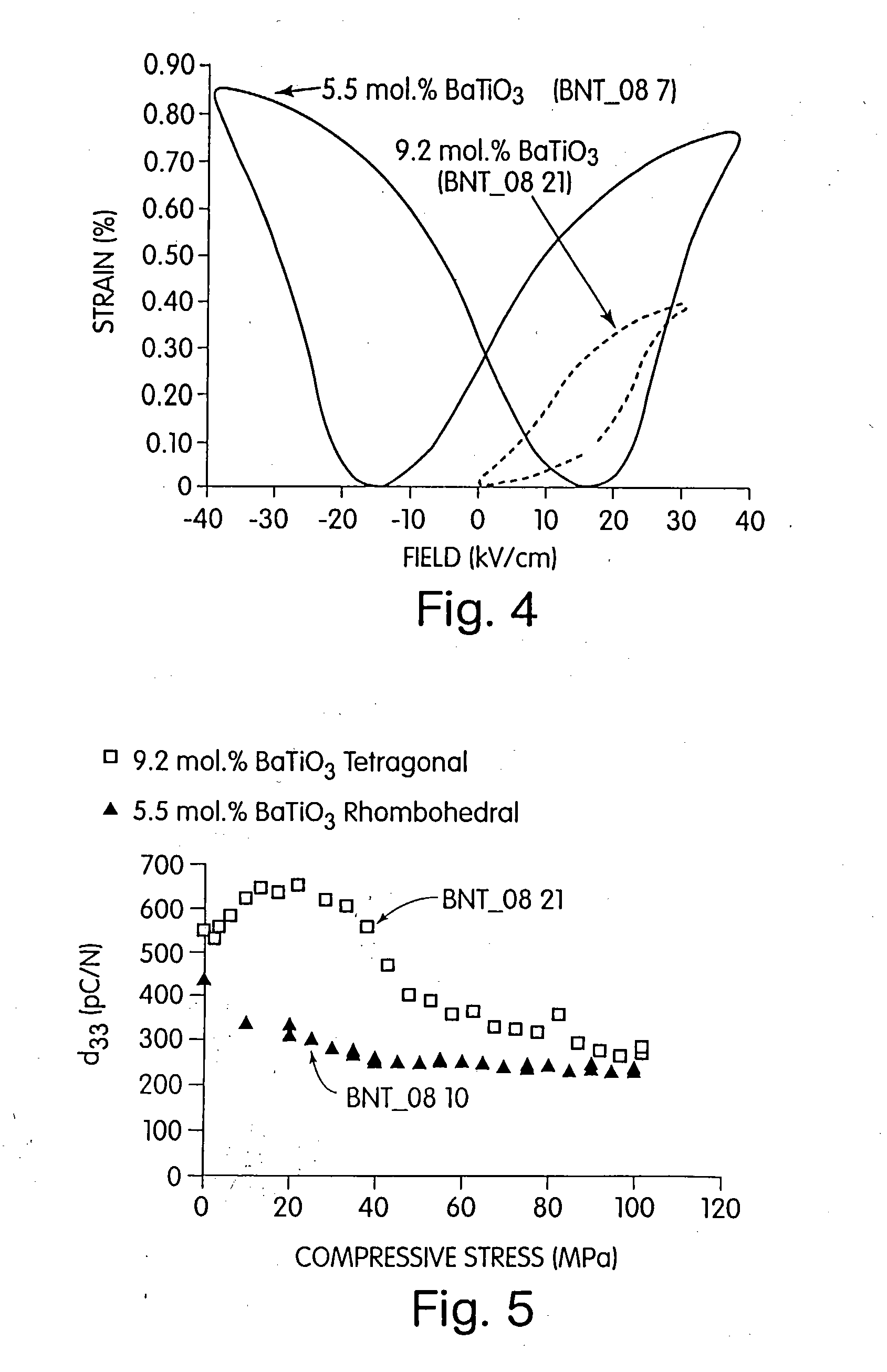
Electromechanical actuators
a technology of electromechanical actuators and perovskite, which is applied in the selection of device materials, non-mechanical valves, lighting support devices, etc., can solve the problems of lead toxicity and associated difficulties in processing, crystal growth and handling, and high density and relatively low elastic modulus of lead based perovskite, etc., to achieve superior electromechanical properties, improve the effect of strain coefficient, and low lead or lead-fr
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Single Crystal Growth in the System Na(1 / 2)Bi(1 / 2)TiO3—BaTiO3.
[0161] A powder mixture of the target composition (0.94)Na(1 / 2)Bi(1 / 2)TiO3-(0.06)BaTiO3 was prepared, with wt % each of Na2CO3 and Bi2O3 being added in excess in order to form a liquid flux. The powder mixture was prepared from the following starting materials in the following amount
CompoundManufacturerAmount (g)CommentBi2O3Morton Thiokol102.340820 wt % excess of thecomponentNa2CO3MCB Reaoents23.276420 wt % excess of thecomponentTiO3NanoTek58.4750BaTiO3Morton Thiokol10.8980
The powders were ball-milled in a 1000 mL polypropylene bottle containing 50% by volume of zirconia milling media and ˜350 mL ethanol for ˜12 hours at a rolling speed of 120 rpm. The mixed powder was dried with a heating lamp, then ground in a mortar and pestle, then placed in an alumina crucible (100 mL) with a cover and calcined in air according to the following heating schedule: 8 hour ramp from room temperature to 800° C., hold for 2 hours, furn...
example 2
Single Crystal Growth in the System Na(1 / 2)Bi(1 / 2)TiO3—K(1 / 2)TiO3—BaTiO3.
[0176] The nominal crystal composition of this batch was (0.782)Na(1 / 2)Bi(1 / 2)TiO3-(0.138)K(1 / 2)Bi(1 / 2)TiO3-(0.08)BaTiO3. A greater amount of excess Bi2O3 and Na2CO3 was added in this growth run than in Example 1. The amounts of each component are given in the table below:
CompoundManufacturerAmount (g)CommentsBi2O3Alfa Aesar73.7132includes 20 wt % of totalNa2CO3Alfa Aesar31.2103includes 20 wt % of totalTiO2Nanotek39.2722K2CO3Alfa Aesar2.3442BaCO3Alfa Aesar0.6209
[0177] The processing conditions were similar to those used for Example 1. A Thermolyne Hi-temp, model 46100 furnace was used. The crystal growth schedule was as follows: 13.5 hour ramp from room temperature to 1350° C., hold for 5 hours, cool slowly at a rate of 5° C. / min to 800° C., then furnace-cool to room temperature. The growth run yielded smaller crystals of cubic habit and approximately 1 mm dimension in a larger amount of crystallized flux. A...
example 3
Textured Crystallites by Cleavage and Capillary Aggregation.
[0180] Several of the Ba-doped NBT crystals of Example 1 were ground coarsely using a mortar and pestle. The resulting powder was mixed with the liquid binder collodion, and spread on a glass slide. The slide was heated for about 10 minutes in a small box furnace to set the glue. During drying, the powder tends to settle against the glass surface, and the capillary action of the liquid also helps to draw the particles together and against the glass surface. After drying, the powder was subjected to powder x-ray diffraction. The x-ray diffraction pattern, FIG. 15, shows stronger intensity for the (202) reflection of the rhombohedral phase ((200) of the corresponding cubic phase) than does a randomly oriented powder. In the powder diffraction pattern for NBT, JCPDS, file 36-0340, the (110) of the rhombohedral phase is expected to have the highest intensity. In our sample, the (202) has the highest intensity, showing that pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com