Non-particulate organic porous material having optical resolution capability and method for manufacturing same
a non-particulate organic porous material and optical resolution technology, applied in the direction of filtration separation, other chemical processes, separation processes, etc., can solve the problems of deterioration of separation performance during continuous operation, small adsorption capacity, and difficult to achieve precise separation, etc., to achieve excellent optical separation capability, limited separation performance, and high physical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
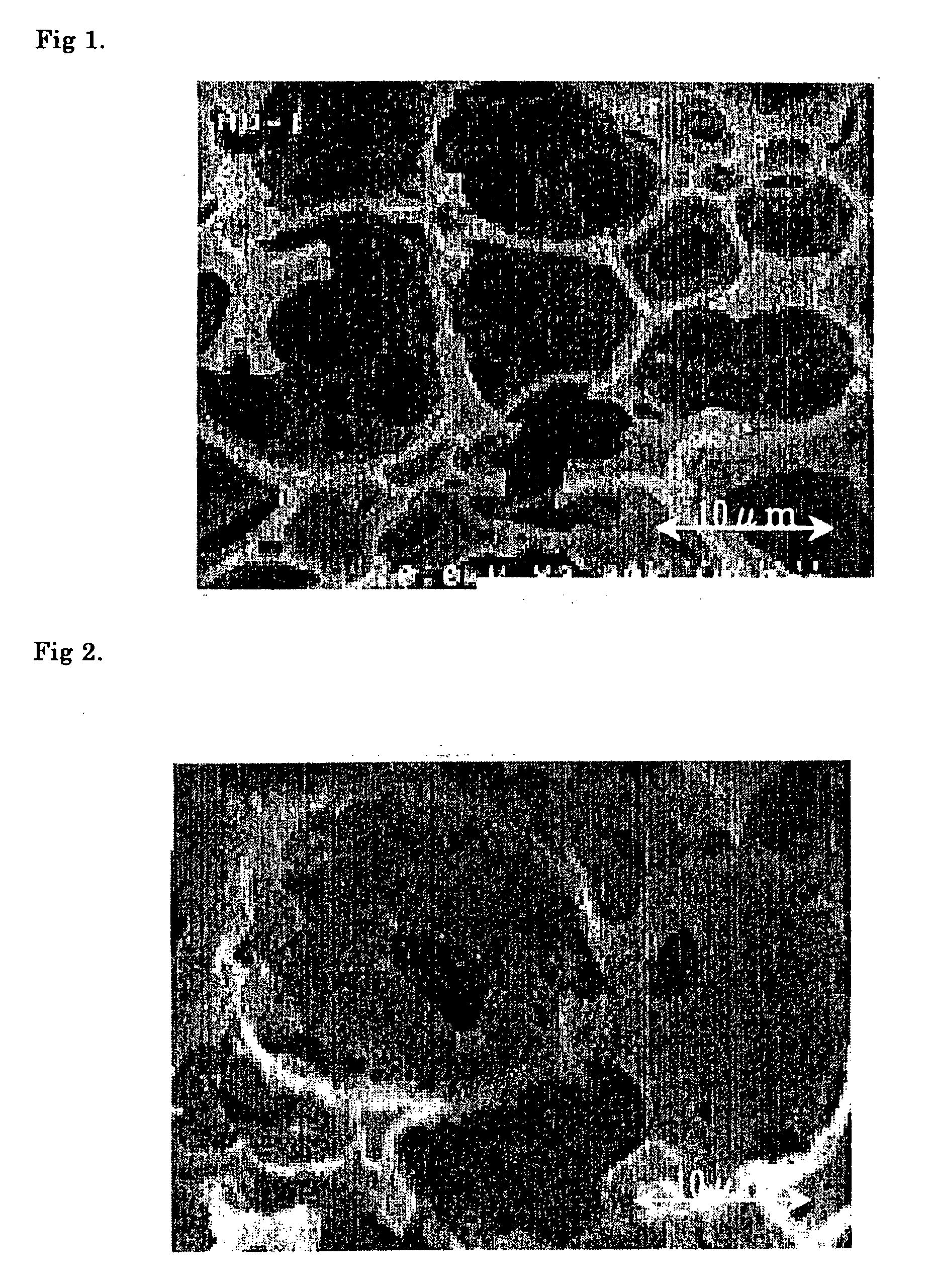
Image
Examples
preparation example 1
Preparation of 4-(−)-pinanyldimethyl silylstyrene (Abbreviated to (−)-PSSt)
[0043] 28.4 g of p-dibromobenzene was dissolved in diethyl ether, and 51.3 g n-butyl lithium was added to react with p-dibromobenzene at 0° C. Then, 31.2 g of (−)-pinanyldimethylsilyl chloride was added and reacted. A mixture obtained after a routine process was purified by distillation under reduced pressure to obtain 1-bromo-4-(−)-pinanyldimethylsilylbenzene. After dissolving 34.85 g of the obtained 1-bromo-4-(−)-pinanyldimethylsilylbenzene in diethyl ether, 42.4 g of n-butyl lithium and 6.9 g of dimethylformamide were successively added and the mixture was reacted. After processing by a routine method, the reaction mixture was purified by silica gel column chromatography to obtain 27.4 g of 4-(−)-pinanyldimethylsilylbenzaldehyde. After dissolving the obtained aldehyde in 200 ml of tetrahydrofuran, the solution was added to a solution of 36.9 g of methyltriphenyl iodide and 40.0 g of n-butyl lithium to ob...
preparation example 2
Preparation of (−)-menthyl 4-vinylbenzoate (Abbreviated to (−)-MtSt)
[0044] 14.8 g of 4-vinylbenzoic acid was dissolved in methylene chloride and reacted with 15.6 g of (−)-menthol, using an N,N′-dicyclohexyl carbodiimide as a condensing agent to obtain (−)-MtSt. The (−)-MtSt was purified by silica gel column chromatography using a hexane / ethyl acetate mixture (19 / 1) as a developing solvent to obtain 20.3 g of purified (−)-MtSt. The structure was confirmed using NMR and IR.
example 1
Preparation of Non-Particulate Organic Porous Material having Optically Active Groups by Introducing (−)-pinanyldimethylsilyl Groups.
[0045] 6.8 g of (−)—PSSt, 2.9 g of divinylbenzene, 1.1 g of sorbitan monooleate, and 0.10 g of azobis-iso-butylonitrile were mixed. A container for emulsion preparation was charged with the mixture and 97 g of deionized water. The resultant mixture was stirred using a sun-and-planettype stirrer (vacuum agitation defoaming mixer, manufactured by EME Co., Ltd.) at a reduced pressure of 13.3 kPa, at a revolution rate (rotation around a revolution axis) of 2,000 rpm, and at a rotation of 600 rpm for 2.5 minutes to obtain a water-in-oil emulsion. After the emulsification, the reaction system ambience was sufficiently replaced with nitrogen and the emulsion was sealed and allowed to stand to polymerize at 60° C. for 24 hours. After the polymerization, the reaction mixture was extracted with isopropanol for 18 hours using a Soxhlet extractor to remove unrea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Pore size | aaaaa | aaaaa |
| Molality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com