Transgenic microbial polyhydroxyalkanoate producers
a technology of polyhydroxyalkanoate and transgenic bacteria, which is applied in the direction of transferases, lyases, enzymology, etc., can solve the problems of pha production of many of the microorganisms in these references that are not commercially useful, p(3hb) production by recombinant organisms is hampered, and the number of plasmid copy numbers is often decreased, so as to achieve the effect of pha production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Host Strains and Plasmid Tools for Gene Integration
[0058] Strains and plasmids from which transposon vectors and transposon derivatives were developed are listed in Tables 2 and 3 below. MBX245 and MBX247 were selected by growing MBX23 and LS5218 respectively on LB plates containing approximately 30 g / ml naladixic acid. MBX246 and MBX248 were selected by growing MBX23 and LS5218, respectively, on LB plates containing 50 g / ml rifampicin. Colonies that appeared on these selective media within 24 hours were replica plated on the same medium and after growth stored in 15% glycerol / nutrient broth at −80° C.
[0059] MBX245 and MBX247 were selected by growing MBX23 and LS5218 respectively on LB plates containing 30 μg / ml naladixic acid. MBX246 and MBX248 were selected by growing MBX23 and LS5218 respectively on LB plates containing 50 μg / ml rifampicin. Colonies that appeared on these selective media within 24 hours were replica plated on the same medium and after growth stored in 15% glyce...
example 2
Construction of Cloning Vectors to Facilitate Integration of phb Genes
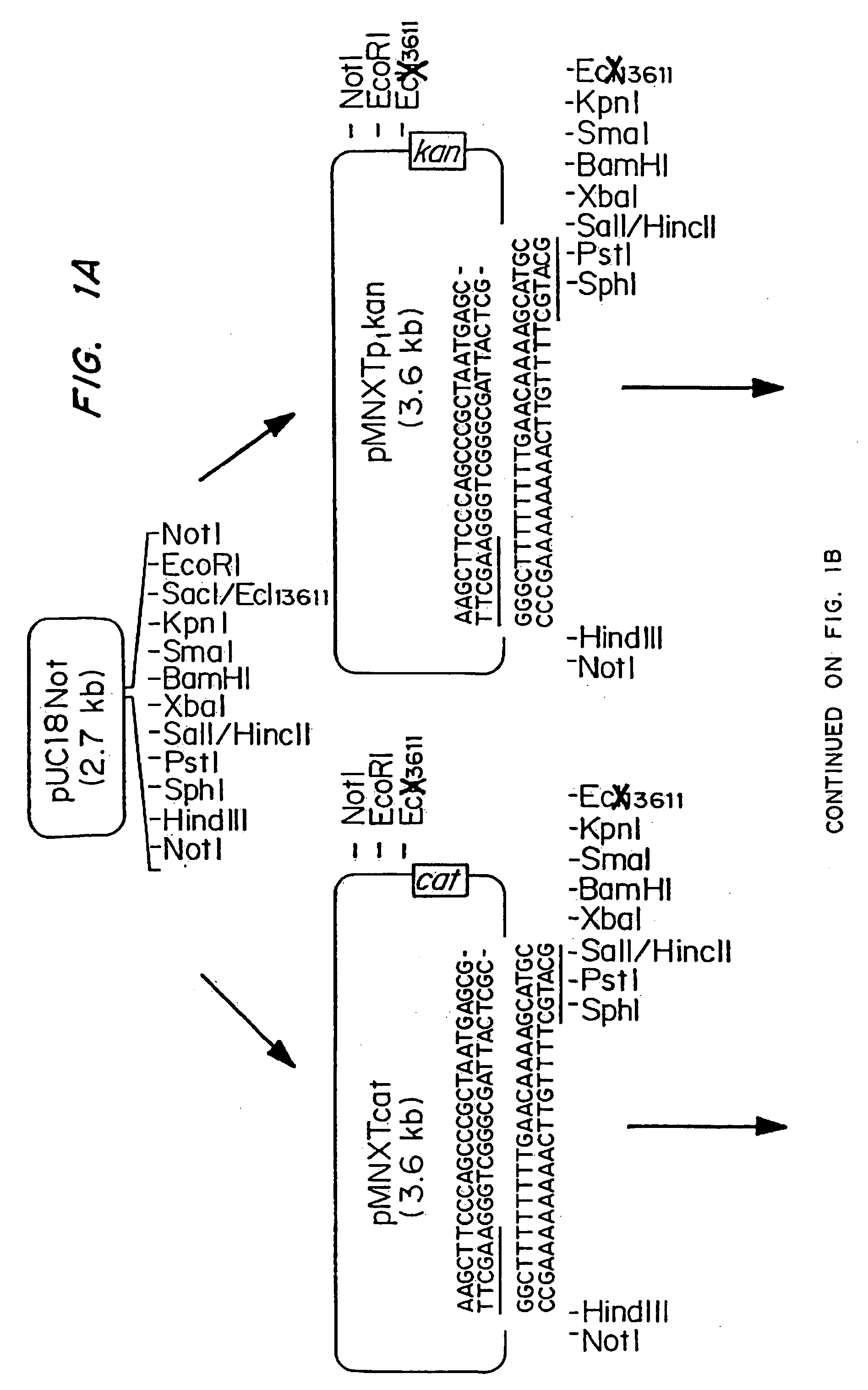
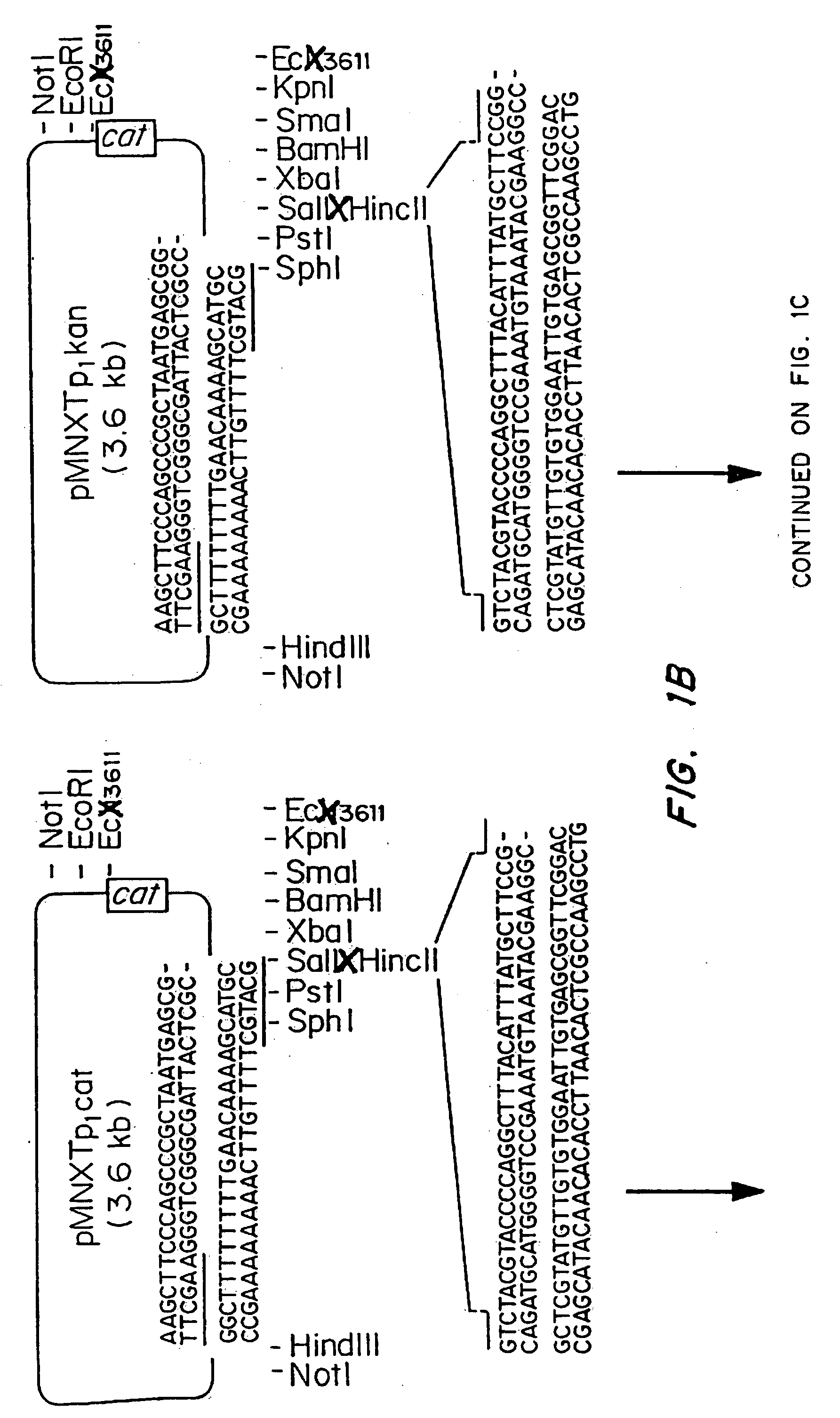
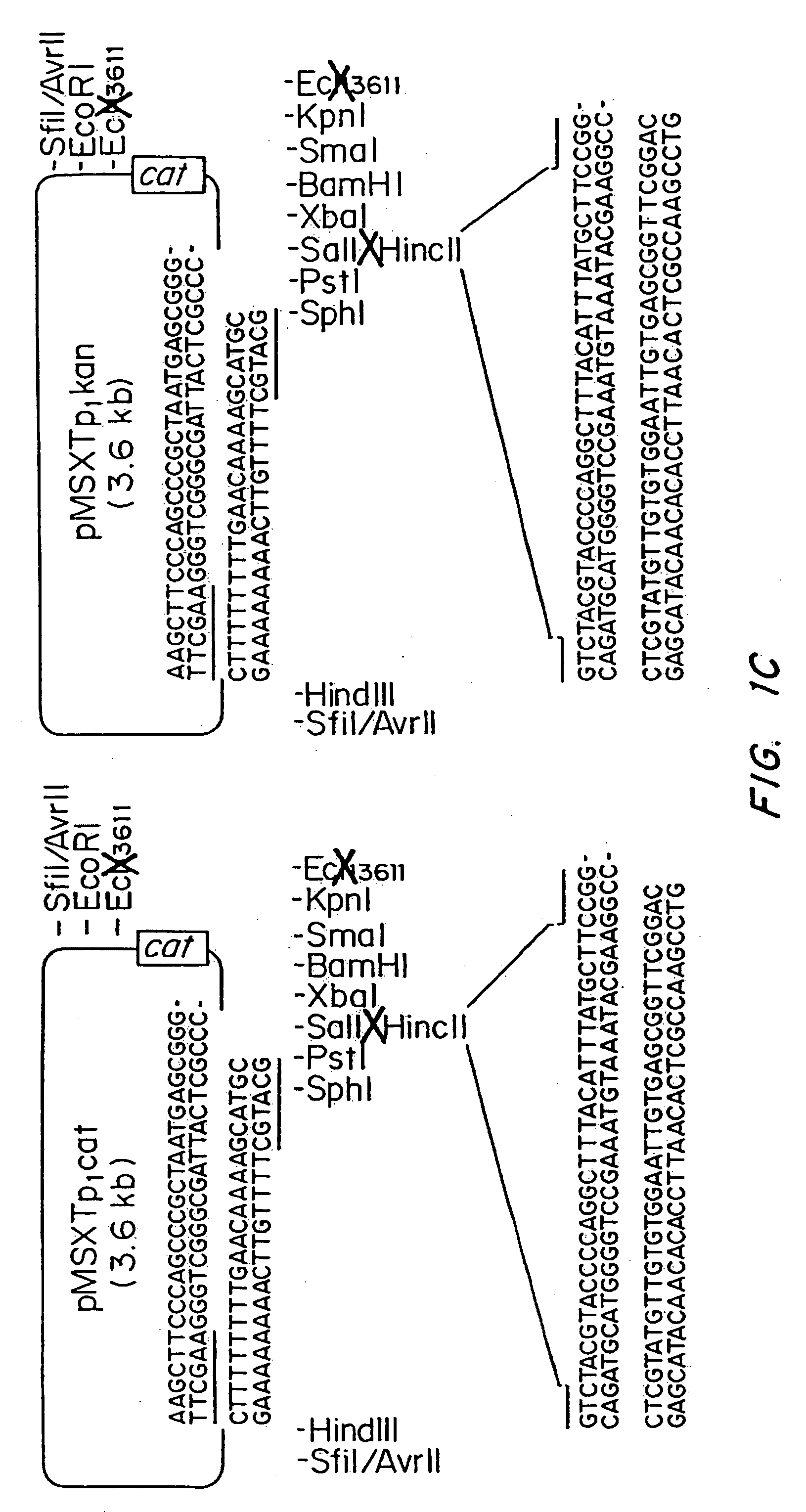
[0061] The plasmids pMNXTp1kan and pMNXTp1cat were based on the plasmids pUC18Not and pUC18Sfi and developed as shown in FIG. 1.
[0062] The Tn903 kanamycin (Km) resistance gene from plasmid pBGS18 was amplified by PCR using the oligonucleotide primers
linkK1,5′ TGCATGCGATATCAATTGTCCA GCCAGAAAGTGAGG,andlinkK2,5′ ATTTATTCAACAAAGCCGCC.
[0063] Prior to PCR amplification, the primers were phosphorylated using T4 polynucleotide kinase using standard procedures. The DNa was amplified using the following program: 1 cycle of 3 min at 95° C., 40 s at 42° C., 2 min at 72° C., followed by 30 cycles of 40 s at 95° C., 40 s at 42° C. and 90 s at 72° C. The DNA then was phenol extracted and treated with T4 DNA polymerase prior to gel purification. The blunt ended 0.8 kb DNA fragment was then inserted into the Ecl136II site in the polylinker of pUC18Not to obtain pMNXkan.
[0064] The cat gene was obtained as an HindIII cassette f...
example 3
Construction of Plasmids for Chromosomal Integration of phbC, Encoding PHB Polymerase
[0068] Plasmid pMUXC5cat contains the phbC gene from Z. ramigera on a transposable element for integration of this gene on the chromosome of a recipient strain, as shown in FIG. 2. Strong translational sequences were obtained from pKPS4 which includes phaC1 encoding PHA polymerase from P. oleovorans in the pTrc vector (Pharmacia). In this construct, phaC1 is preceded by a strong ribosome binding site: AGGAGGTTTTT(-ATG). The phaC1 gene including the upstream sequences, was cloned as a blunt ended EcoRI-HindIII fragment in the SmaI site of pUC18Sfi to give pMSXC3. A blunt ended cat gene cassette was subsequently cloned in the blunt-ended Sse8387II site, resulting in pMSXC3cat. At this point, all of the phaC1 coding region except the 5′ 27 base pairs were removed as a PstI-BamHI fragment and replaced by the corresponding fragment from the phbC gene from Z. ramigera. The resulting plasmid pMSXC5cat enc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cell density | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com