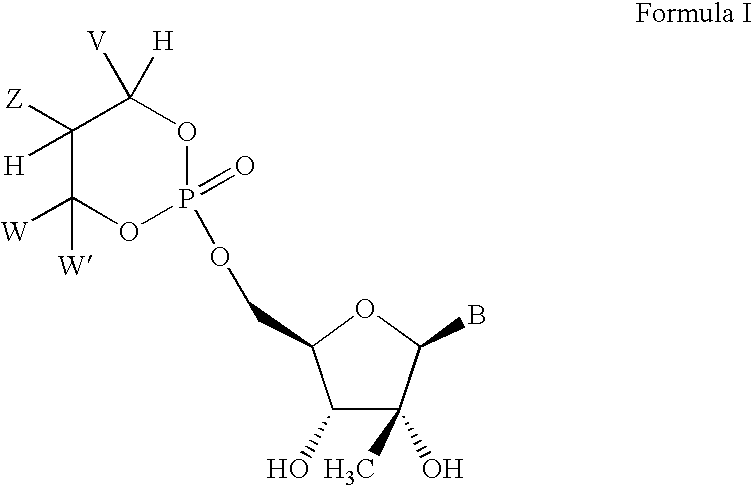
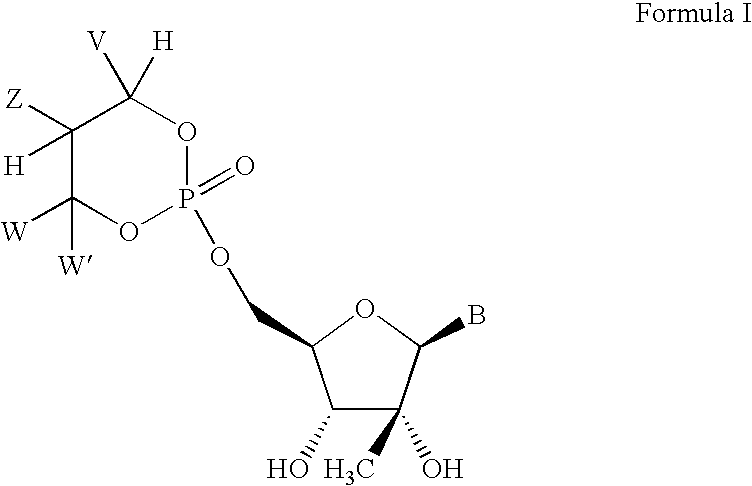
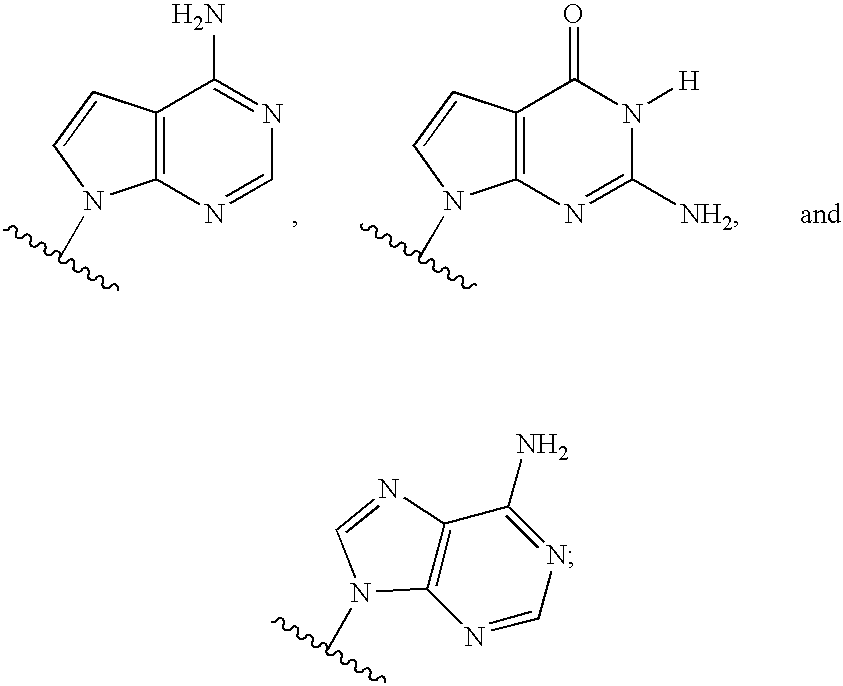
Novel 2'-C-methyl nucleoside derivatives
a technology of c-methyl nucleosides and derivatives, which is applied in the direction of sugar derivatives, antivirals, organic chemistry, etc., can solve the problems of poor kinase substrates, complex use of nucleosides for viral liver infections, and poor efficacy, so as to improve drug efficacy or safety, improve oral bioavailability, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1-(2′-Furanyl)-Propane-1,3-Diol via Grignard Addition and Hydroboration
[0558] To a solution of 2-furaldehyde (3 g, 31.2 mmol) in THF (60 mL) was added 1 M vinyl magnesium bromide in THF (34 mL) at 0° C. After stirring for an hour, a solution of 1 M BH3 THF complex in THF was added. The reaction was quenched with 3N NaOH (20 mL) and 30% hydrogen peroxide (10 mL) at 0° C. The organic fraction was separated and concentrated. The crude product was chromatographed by eluting with 5% methanol-dichloromethane to give 1-(2′-furyl)propane-1,3-diol (1 g).
example 2
Preparation of 1-(2′-Pyridyl)-Propane-1,3-Diol Via Benzylic Oxidation
Step A: (J. Org. Chem. 22:589 (1957))
[0559] To a solution of 3-(2′-pyridyl)propan-1-ol (10 g, 72.9 mmol) in acetic acid (75 mL) was added 30% hydrogen peroxide slowly. The reaction mixture was heated to 80° C. for 16 h. The reaction was concentrated under vacuum and the residue was dissolved in acetic anhydride (100 mL) and heated at 110° C. overnight. Acetic anhydride was evaporated upon completion of the reaction. Chromatography of the mixture by eluting with methanol-methylene chloride (1:9) resulted in 10.5 g of pure diacetate.
Step B:
[0560] To a solution of diacetate (5 g, 21.1 mmol) in methanol-water (3:1, 40 mL) was added potassium carbonate (14.6 g, 105.5 mmol). After stirring for 3 h at room temperature, the reaction mixture was concentrated. The residue was chromatographed by eluting with methanol-methylene chloride (1:9) to give 2.2 g of crystalline diol.
example 3
Preparation of 1-(Aryl)-Propane-1,3-Diol from Propane-1,3-Diol Via Grignard Addition
Step A: (J. Org. Chem. 53:911 (1988))
[0561] To a solution of oxalyl chloride (5.7 mL, 97 mmol) in dichloromethane (200 mL) at −78° C. was added dimethyl sulfoxide (9.2 mL, 130 mmol). The reaction mixture was stirred at −78° C. for 20 min before addition of 3-(benzyloxy)propan-1-ol (11 g, 65 mmol) in dichloromethane (25 mL). After an hour at −78° C., reaction was quenched with triethylamine (19 mL, 260 mmol) and warmed to room temperature. Work-up and column chromatography by elution with dichloromethane resulted in 8 g of 3-(benzyloxy)propan-1-al.
Step B:
[0562] To a solution of 3-(benzyloxy)propan-1-al (1 g, 6.1 mmol) in THF at 0° C. was added a 1 M solution of 4-fluorophenylmagnesium bromide in THF (6.7 mL, 6.7 mmol). The reaction was warmed to room temperature and stirred for 1 h. Work-up and column chromatography by elution with dichloromethane resulted in 0.7 g of alcohol.
Step C:
[0563] To...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com