Curing accelerator for curing resin, curing resin composition, electronic component device and method for producing phosphine derivative
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
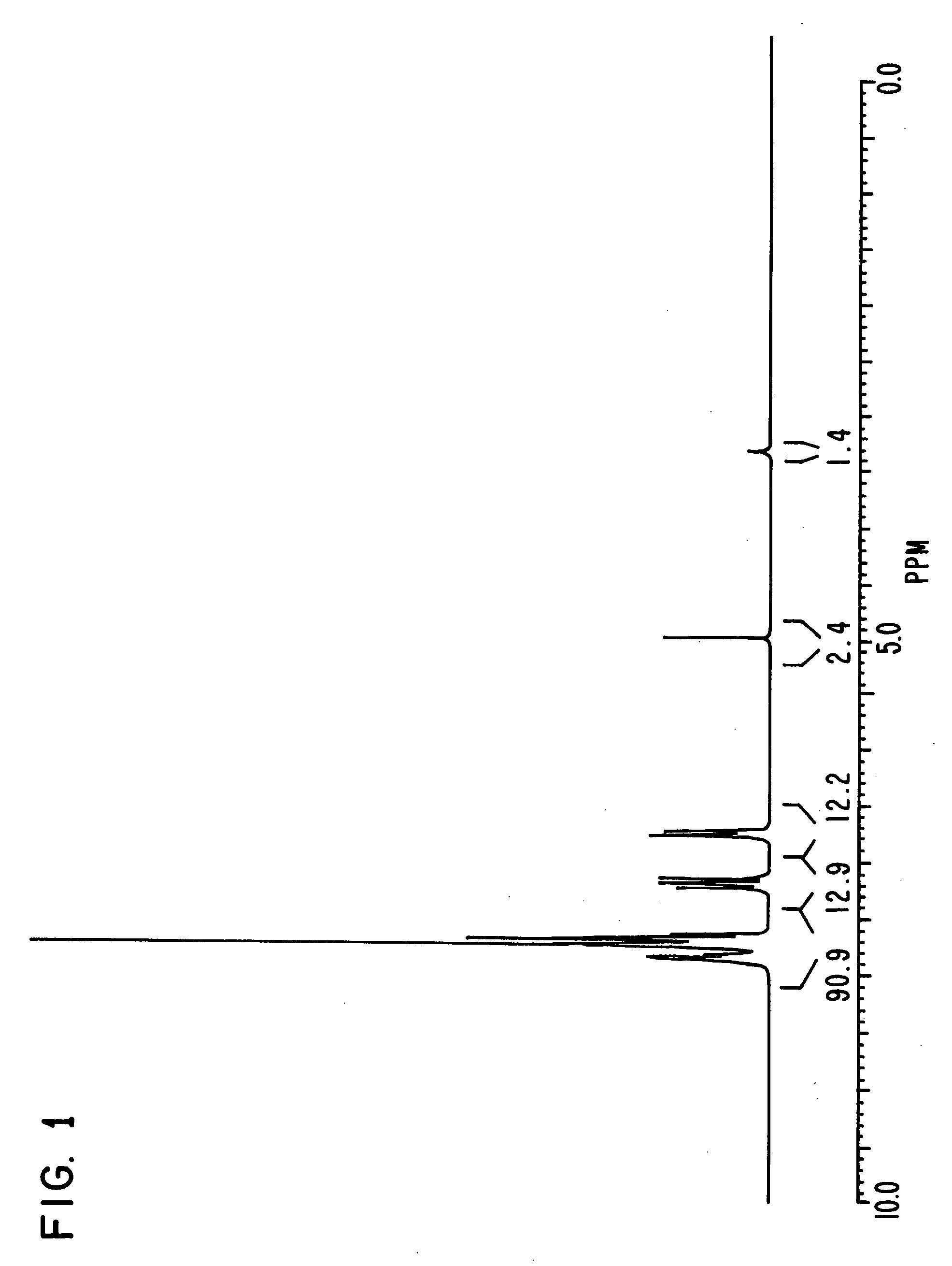
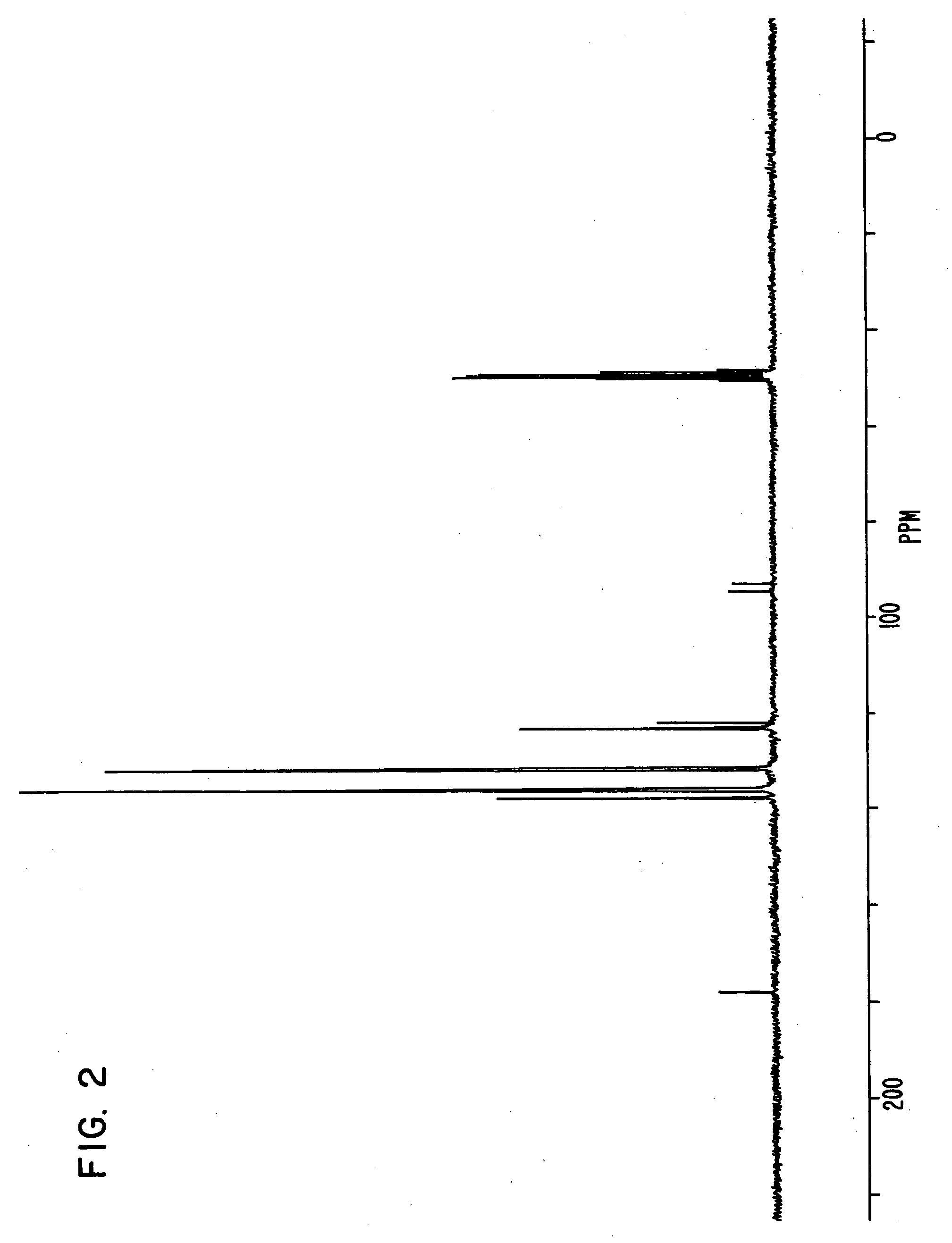
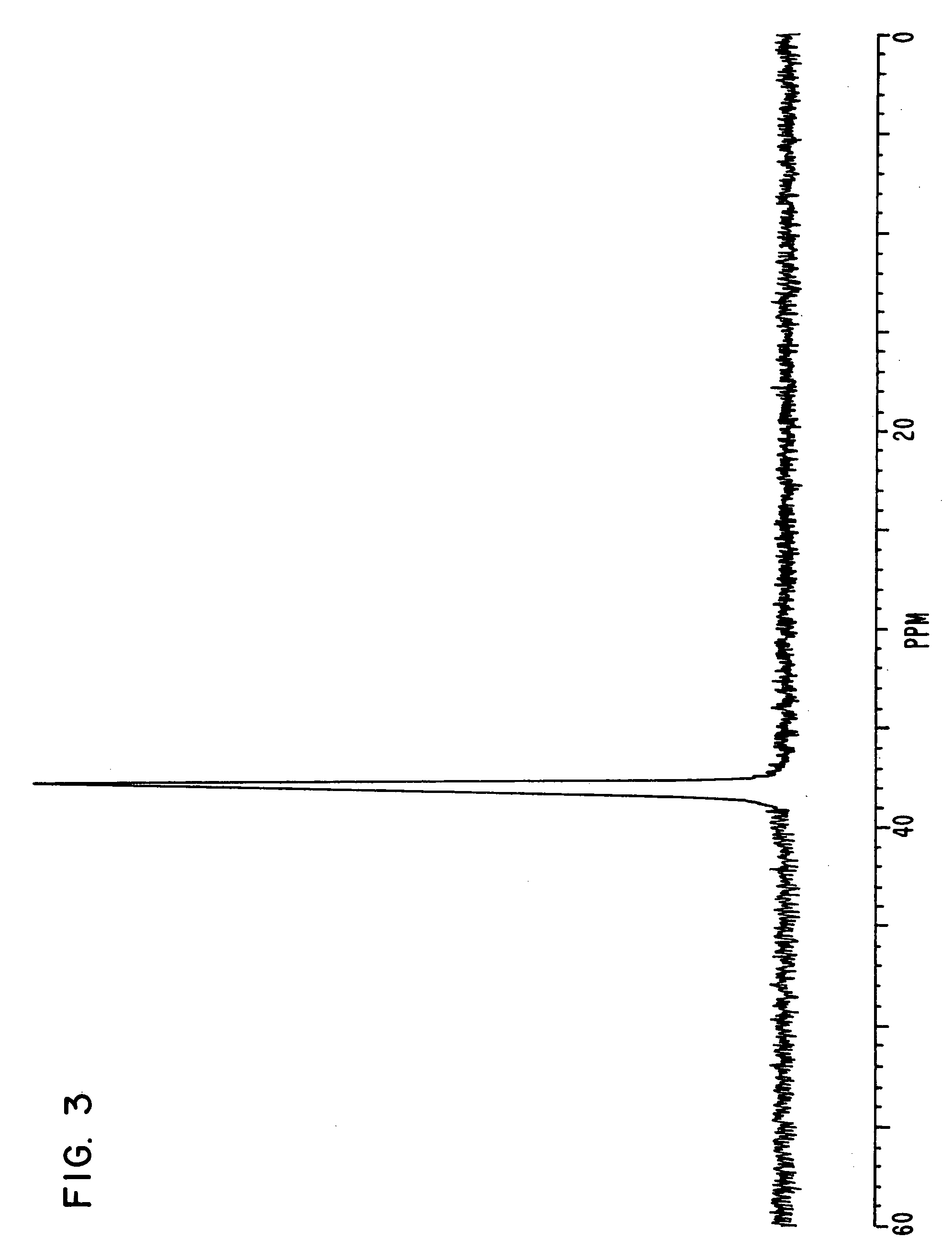
[0170] Triphenylphosphine of 20.4 g, 4-bromo phenol of 26.9 g, nickel (II) chloride hexahydrate of 3.5 g and DMF of 20 g were put in a flask, and were stirred at 145° C. for 6 hours. Under reduced pressure, the reaction liquid was concentrated, and methanol of 60 ml was added to the reaction liquid. Sodium hydroxide of 9.3 g was then added to the reaction liquid, and the reaction liquid was stirred until the sodium hydroxide was completely dissolved.
[0171] The solution obtained was filtered on celite, and was concentrated under reduced pressure until the whole amount became about 50 ml. The solution was then turned on into water of 1 liter, and the crystal deposited was filtered. The crystal was then dried under reduced pressure after washing, and a compound of 25.6 g was obtained. Elementary analysis revealed that C was 81.34 and H was 5.40 as calculated values (%), and C was 81.21 and H was 5.34 as measured values (%).
synthesis example 2
[0172] A compound of 24.5 g was obtained in the same manner as in Synthesis Example 1 except that 4-chlorophenol of 20 g was put in place of 4-bromo phenol. Elementary analysis revealed that C was 81.34 and H was 5.40 as calculated values (%), and C was 81.23 and H was 5.33 as measured values (%).
synthesis example 3
[0173] Triphenylphosphine of 20.4 g, 3-bromo phenol of 26.9 g, nickel (II) chloride hexahydrate of 3.5 g and DMF of 20 g were put in a flask, and were stirred at 145° C. for 6 hours. Under reduced pressure, the reaction liquid was concentrated, and methanol of 60 ml was added to the reaction liquid. Sodium hydroxide of 9.3 g was then added to the reaction liquid, and the reaction liquid was stirred until the sodium hydroxide was completely dissolved. The solution obtained was filtered on celite, and was concentrated under reduced pressure until the whole amount became about 50 ml. The solution was then turned on into water of 1 liter. The resultant solution was concentrated until the resultant solution become about 200 ml, and the crystal deposited was filtered. The crystal was then dried, and a compound of 10.2 g was obtained. Elementary analysis revealed that C was 81.34 and H was 5.40 as calculated values (%), and C was 81.15 and H was 5.29 as measured values (%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com