Hazard-free microencapsulation for structurally delicate agents, an application of stable aqueous-aqueous emulsion
a technology of structurally delicate agents and microencapsulation, applied in the direction of spray delivery, peptide/protein ingredients, aerosol delivery, etc., can solve the problems of protein denaturement during the formulation process, sustained release or non-invasive, etc., to reduce the portion of incomplete release, prevent burst effect, and rapid release of considerable amount of loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Stability of Polymer Aqueous-aqueous Emulsion
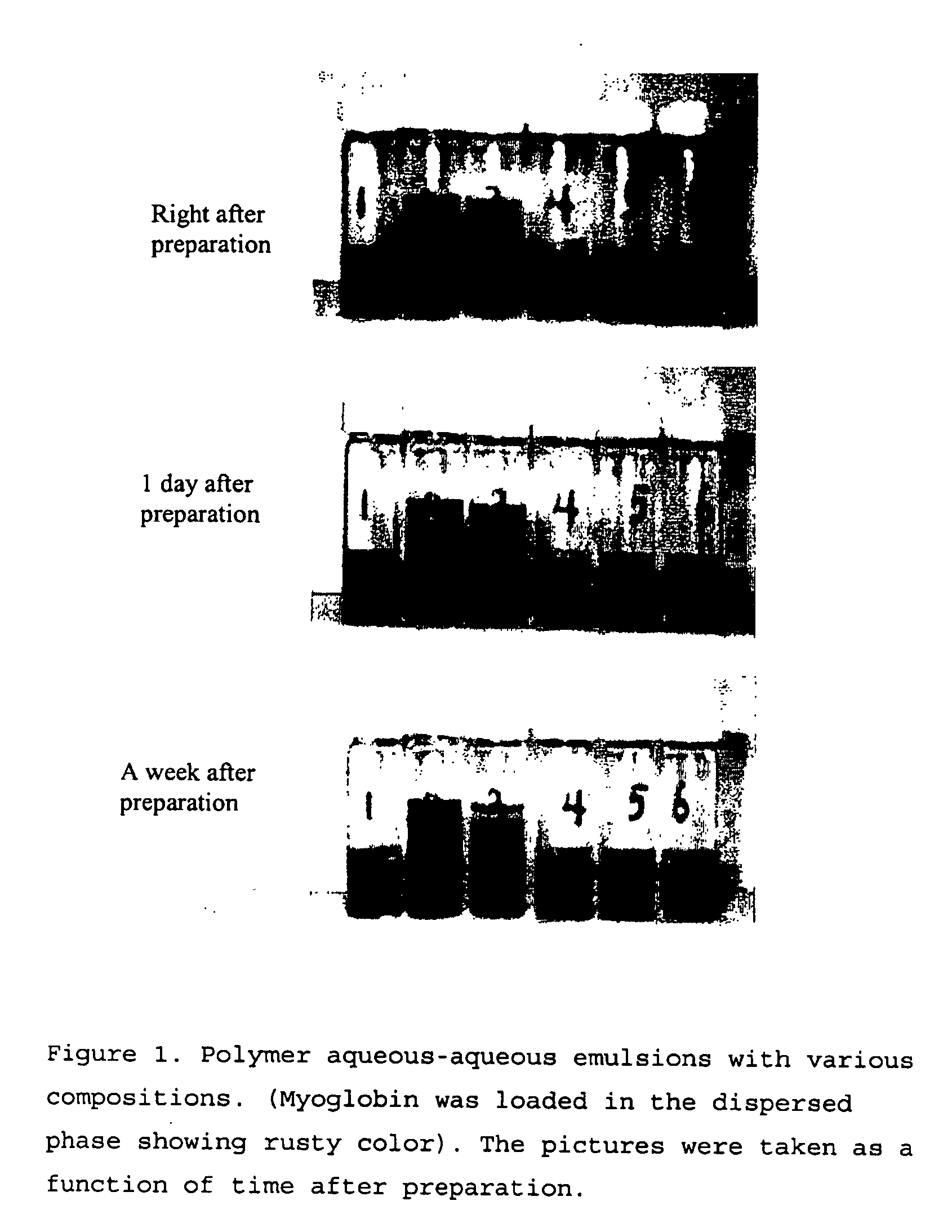
[0056] Stability of polymer aqueous-aqueous emulsion was examined by observation of the fusion (the size change) of the dispersed phase under a microscope and by observation of formation of block phases of the colored dispersed phase directly by eyes as a function of time. The dispersed phase was formed by a dextran solution. Three concentrations of the dextran solution, 5, 20 and 40 w / w %, were used in the experiments without significant difference in the results, i.e. for either of the concentration, stable aqueous-aqueous emulsion was formed. For the average molecular weight of dextran, W=10,000, 67,000 and 500,000 were tested without significant difference in results. The continuous phase contained PEG with concentration 5, 20, and 40 w / w % in different tests, for all of which, stable emulsion was formed. Average molecular weight of PEG used were 8000 and 22,000. As emulsion stabilizers, sodium alginate, carboxymethyl dextran, carbox...
example 2
Preparation of AqueSpheres
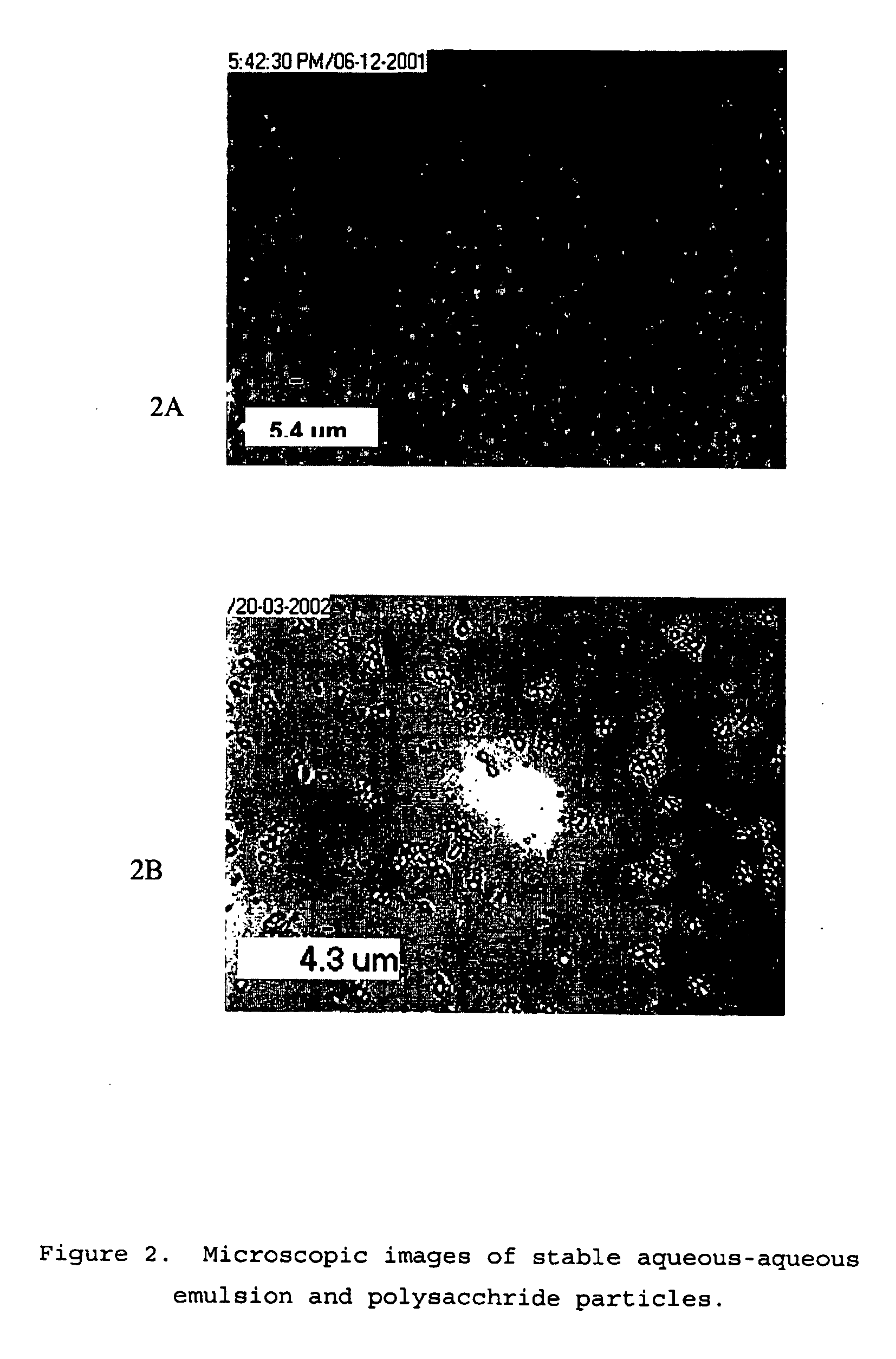
[0061] AqueSpheres were prepared simply by freeze-drying the stable emulsions of above. After freeze-drying, the dextran droplets converted to solid particles. However, the most of dextran particles were dispersed in a solid matrix formed by the continuous phase, PEG. The PEG can be removed by washing the lyophilized powder with methylene chloride or acetonitrile. These solvents did neither dissolve nor swell the dried dextran phase. FIGS. 2A and 2B showed the microscopic images of the dispersed phase at different preparation stages: after emulsification, after freeze-drying followed by rinsing with dichloromethane (to remove PEG), and after recovery from PLGA coating, respectively. After freeze-drying, the diameter of the dispersed phase remained uniform but dropped from 3-7 μm to 1-3 μm, a reasonable size reduction from loss of water (See FIG. 2B). These images indicated that no droplet fusion occurred during lyophilization. This size range of the dried ...
example 3
Microencapsulation of AqueSpheres into PLGA Microspheres
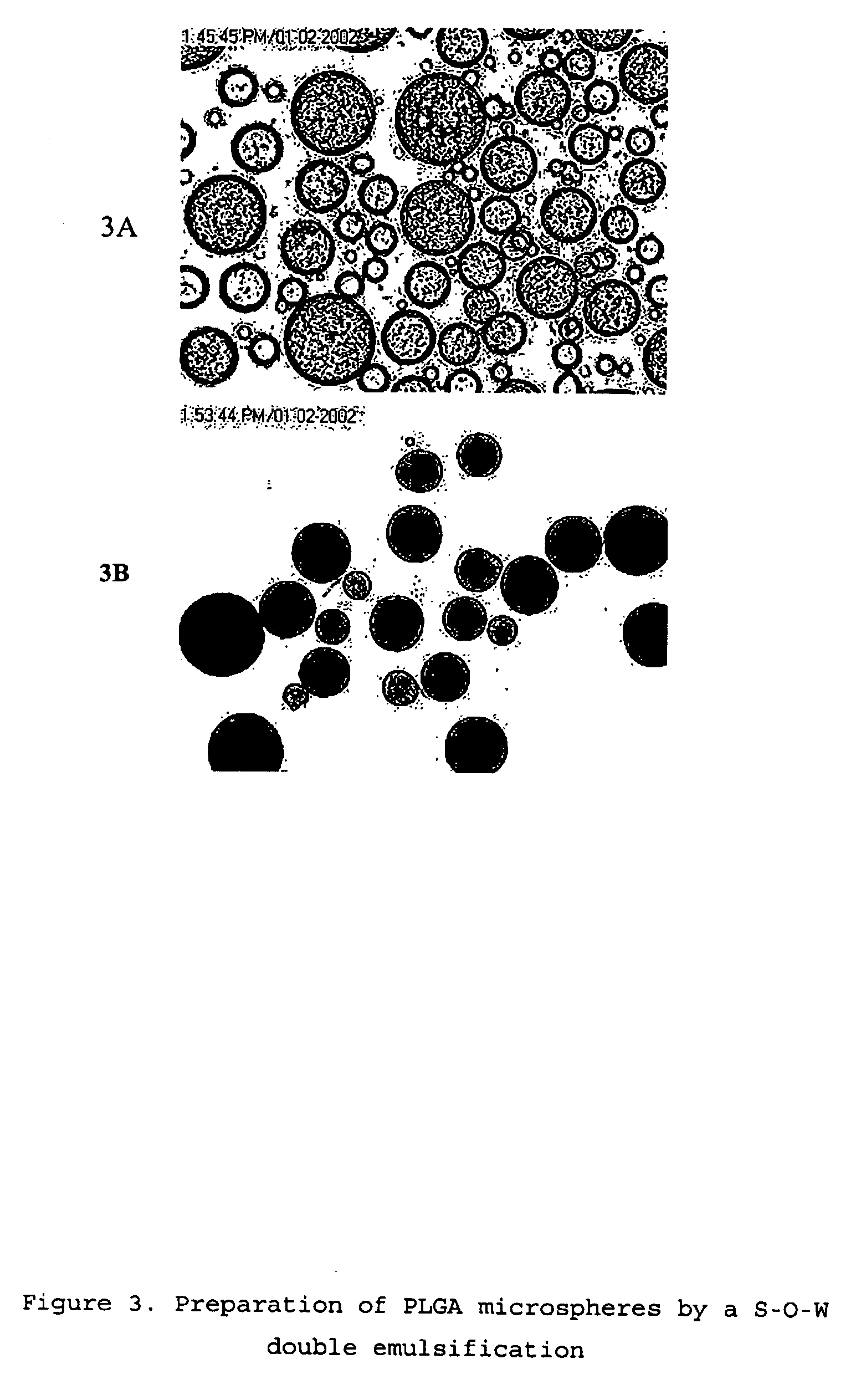
[0062] AqueSpheres can be further microencapsulated into the matrix of PLGA and other biodegradable polymer microspheres through a “solid-in-oil-in-water” emulsification process. In the present study, PLGA with lactic:glycolic ratio of 50:50 and 75:25 were used. AqueSpheres prepared as in Example 2 were first suspended in a PLGA / dichloromethane solution (10-20%) at the AqueSphere / PLGA ratio of 1:2 to 1:20, then added into a water solution containing 0.1-10% sodium chloride and 0.1-4% polyvinyl alcohol (PVA) or PEG or polyvinyl parralidone (PVP) under stirring. The volume ratio of the two solutions was 1:2 to 1:10. After an emulsion was formed, the organic solvent was extracted by pouring the system into large volume of cold water (10 times of the emulsion) under stirring. FIGS. 3A and 3B show the microscopic images of the PLGA droplets before solvent extraction and PLGA particles after solvent extraction, respectively. Before ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com