Olefin wax, silicone-modified olefin waxes, silicone-base cold-setting compositions made by using the same, and cosmetics containing the same
a technology of silicone-modified olefin wax and olefin wax, which is applied in the field of olefin wax and silicone-modified olefin wax, can solve the problems of insufficient performance of silicone-modified polyethylene wax, inability to obtain homogeneous silicone-modified polyethylene wax, etc., and achieves excellent usability, light spreading feeling, and strong repellency against sweat and water.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
(Example 2 of the Metallocene Compound)
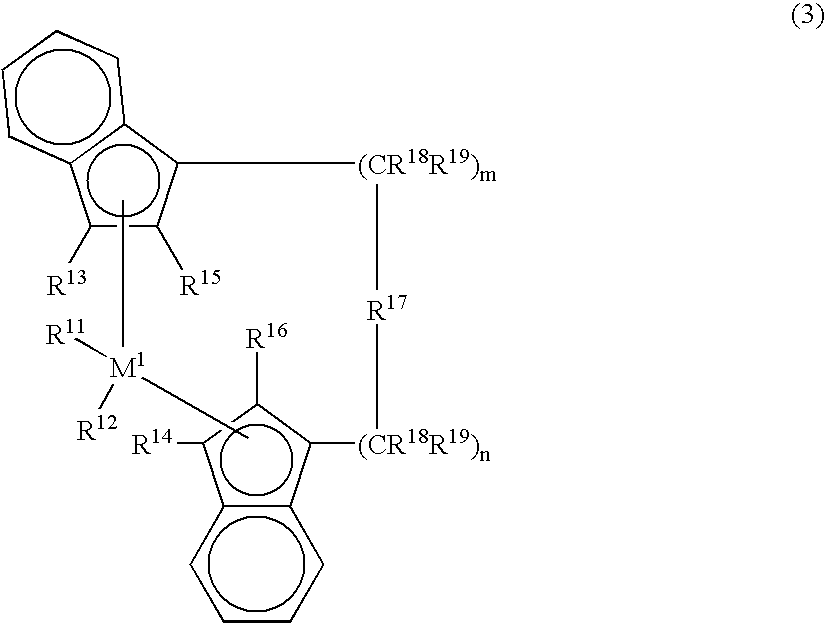
[0061] Another example of the metallocene compound is a metallocene compound represented by the following formula (3) that is described in Japanese Patent Laid-Open Publication No. 4-268307 / 1992.
[0062] In the formula, M1 is a transition metal of Group 4 of the periodic table, specifically, titanium, zirconium or hafnium. R11 and R12 are each independently a hydrogen atom, an alkyl group of 1 to 10 carbon atoms, an alkoxy group of 1 to 10 carbon atoms, an aryl group of 6 to 10 carbon atoms, an aryloxy group of 6 to 10 carbon atoms, an alkenyl group of 2 to 10 carbon atoms, an arylalkyl group of 7 to 40 carbon atoms, an alkylaryl group of 7 to 40 carbon atoms, an arylalkenyl group of 8 to 40 carbon atoms or a halogen atom. R11 and R12 are each preferably a chlorine atom.
[0063] In the formula (3), R13 and R14 are each independently a hydrogen atom, a halogen atom, an alkyl group of 1 to 10 carbon atoms which may be halogenated, an aryl group of...
example 3
(Example 3 of the Metallocene Compound)
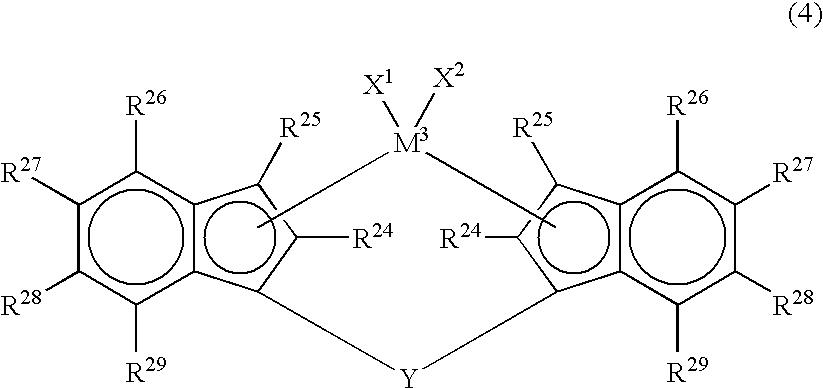
[0068] As the metallocene compound, a metallocene compound represented by the following formula (4) is also employable.
[0069] In the formula (4), M3 is a transition metal atom of Group 4 of the periodic table, specifically, titanium, zirconium or hafnium. R24 and R25 are each independently a hydrogen atom, a halogen atom, a hydrocarbon group of 1 to 20 carbon atoms, a halogenated hydrocarbon group of 1 to 20 carbon atoms, a silicon-containing group, an oxygen-containing group, a sulfur-containing group, a nitrogen-containing group or a phosphorus-containing group. R24 is preferably a hydrocarbon group and is particularly preferably an alkyl group of 1 to 3 carbon atoms, such as methyl, ethyl or propyl. R25 is preferably a hydrogen atom or a hydrocarbon group and is particularly preferably a hydrogen atom or an alkyl group of 1 to 3 carbon atoms, such as methyl, ethyl or propyl. R26, R27, R28 and R29 are each independently a hydrogen atom, a h...
example 4
(Example 4 of the Metallocene Compound)
[0071] As the metallocene compound, a metallocene compound represented by the following formula (5) is also employable.
[0072] In the formula (5), M3, R24, R25, R26, R27, R28 and R29 have the same definitions as those in the formula (4). Of R26, R27, R28 and R29, two groups including R26 are each preferably an alkyl group, and “R26 and R28” or “R28 and R29” are preferably alkyl groups. This alkyl group is preferably a secondary or tertiary alkyl group, and may be an alkyl group substituted with a halogen atom or a silicon-containing group. Examples of the halogen atoms and the silicon-containing groups include the substituents previously exemplified for R24 and R25. Of R26, R27, R28 and R29, a group other than an alkyl group is preferably a hydrogen atom. Two groups selected from R26, R27, R28 and R29 may be bonded to each other to form a monocyclic or polycyclic ring other than an aromatic ring. Examples of the halogen atoms include the same ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com