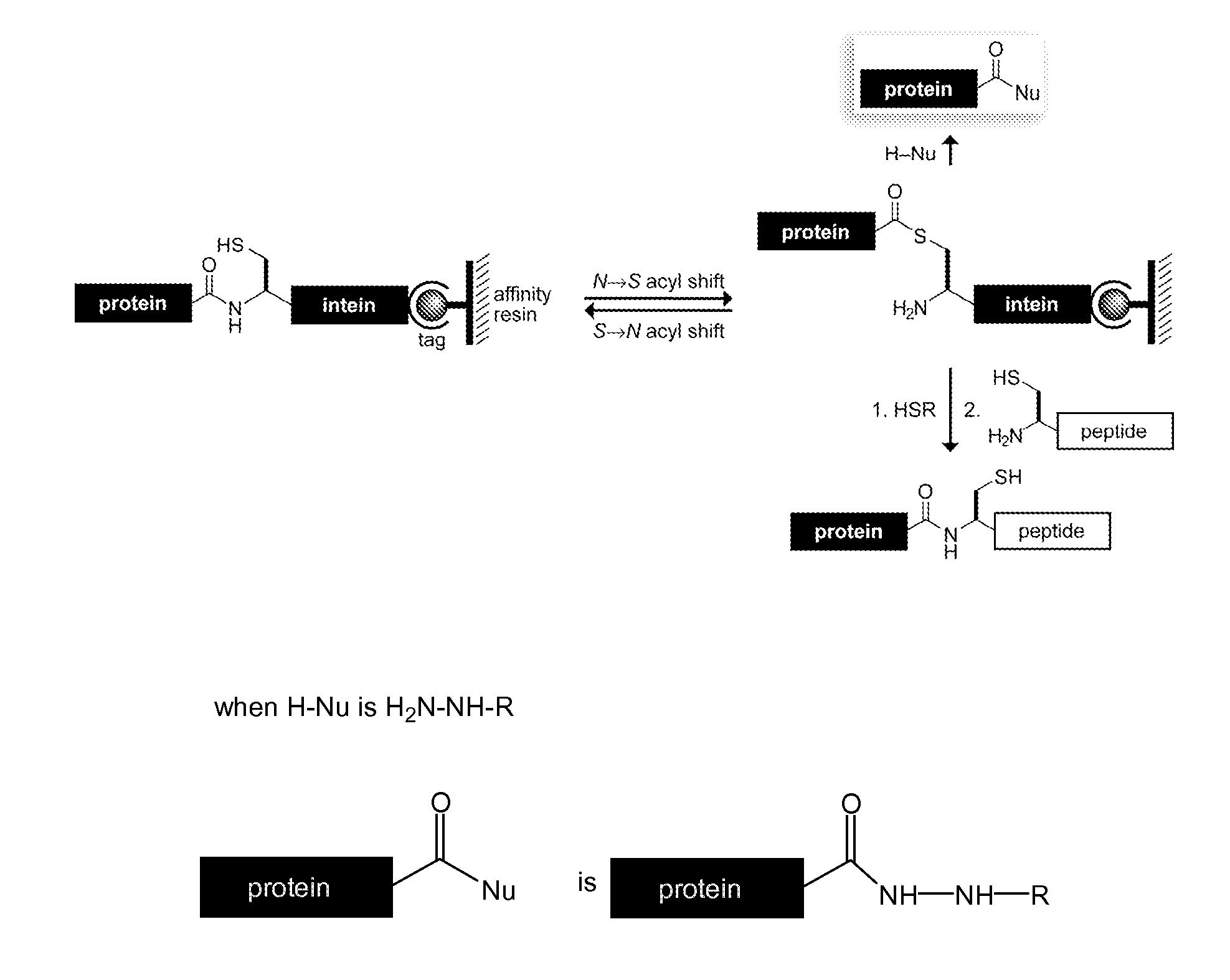
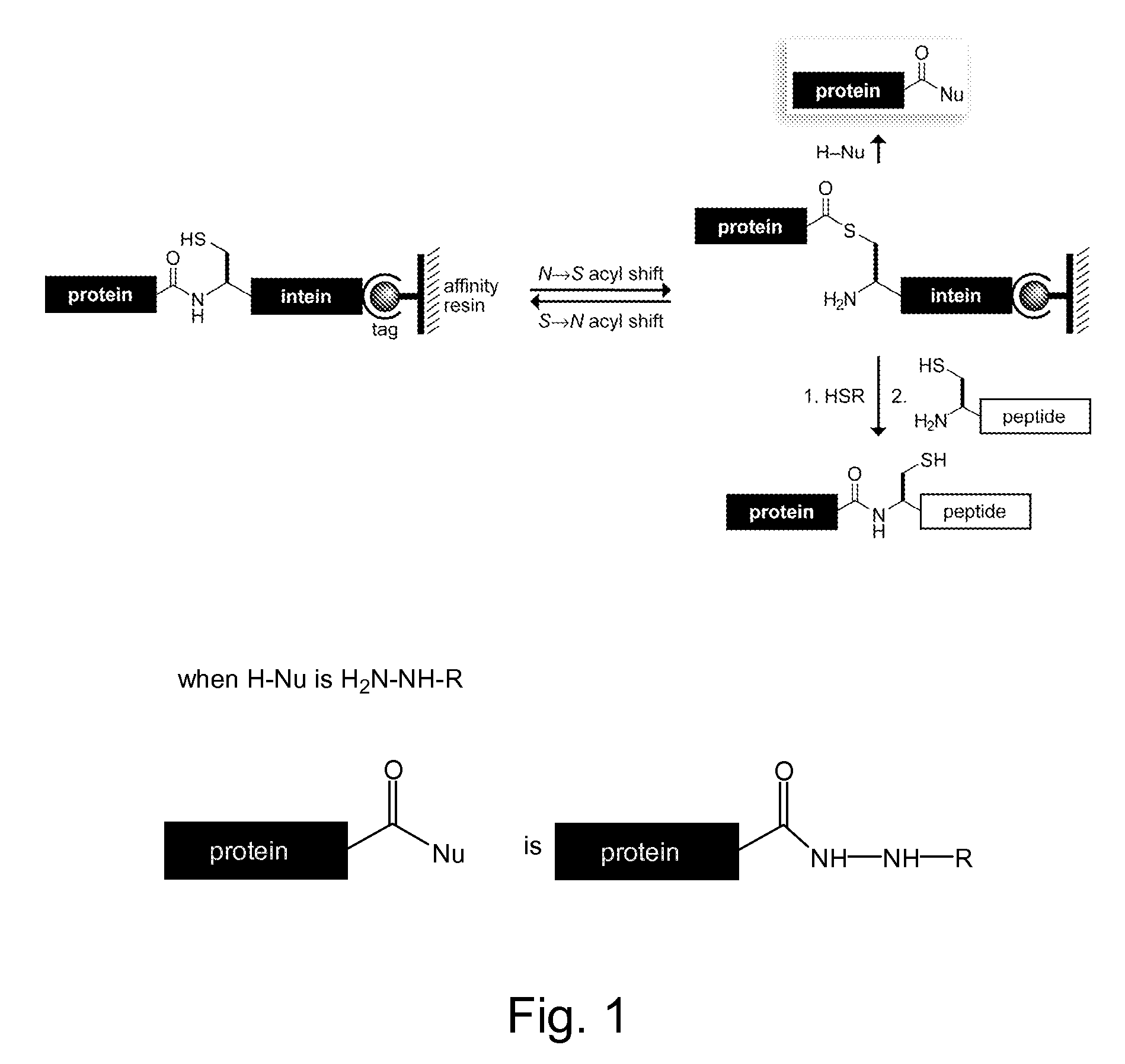
Reagents and Methods for Appending Functional Groups to Proteins
a functional group and protein technology, applied in the field of proteins and functional groups appending, can solve the problems of protein stability, protein stability is limited, and the fabrication of protein microarrays is more arduous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of the Optimal Nucleophile for Thioesters
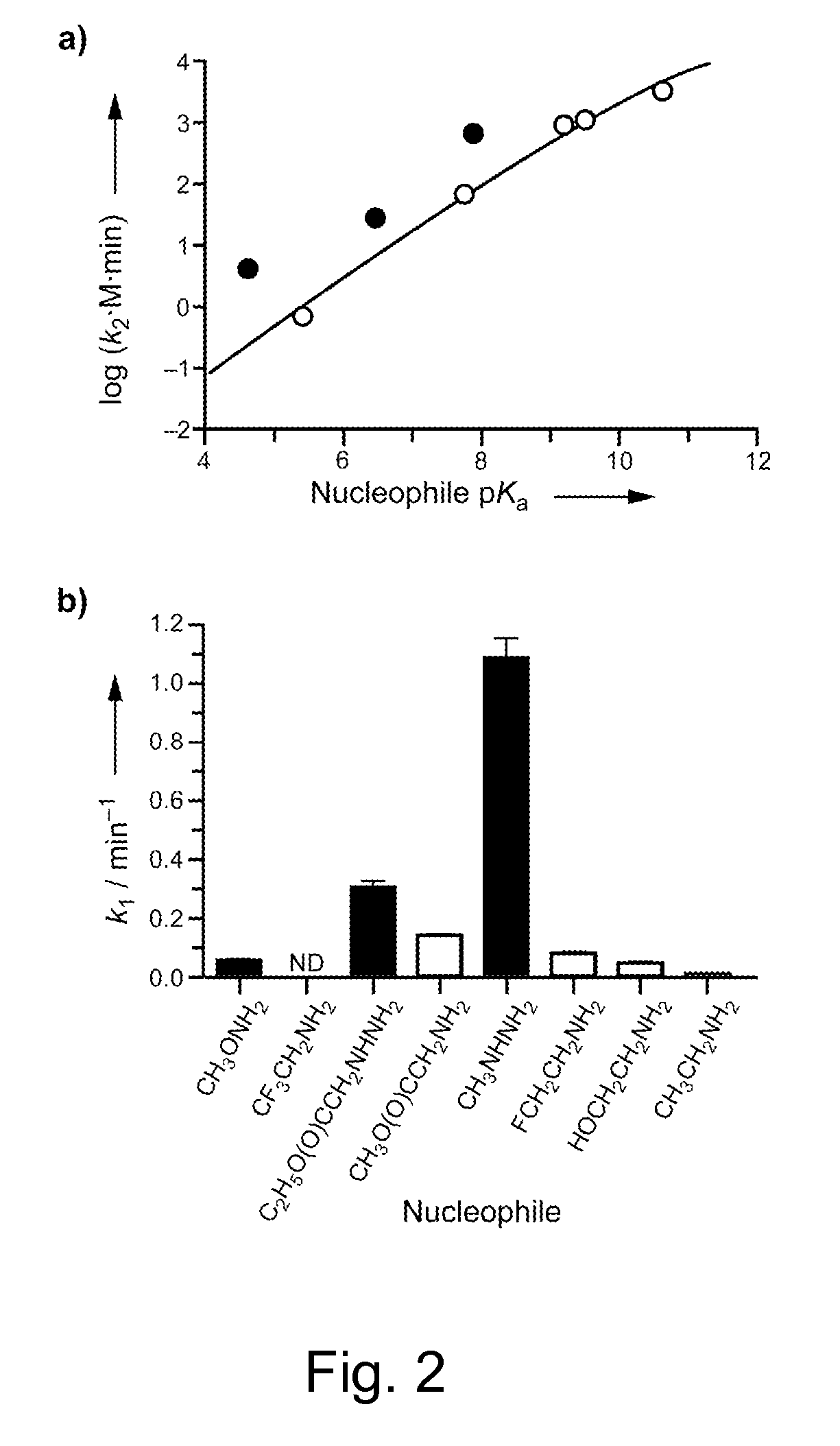
[0107]To identify the optimal nitrogen nucleophile for a thioester, kinetic studies were performed on a model chromogenic thioester: AcGlySC6H4-p-NO2. The rate of release of the thiophenolate anion was monitored by measuring the change in absorbance at 410 nm (Scheme 1). Nitrogen nucleophiles with conjugate-acid pKa values ranging from 4.6 to 10.6 (Table 1) were used in the experiments. The logarithmic values of the second-order rate constants (k2) of the unprotonated primary amines were plotted against the pKa values of their conjugate acids to yield the Brønsted plot shown in FIG. 2A. The data were fitted to the equation[26]:
log k2=log(AB)+(β+β′)pKa−log(A10βpKa+B10β′pKa) (1)
In eq 1, A and B are constants, β′ is the slope of the former part of the Brønsted plot, and β is the slope of the latter part.
[0108]
TABLE 1Nucleophiles used in this studyand the pKa values of their conjugate acids.NucleophilepKaCH3ONH24.60[22]F3CCH2NH25....
example 2
Synthesis of Bifunctional Azides
[0112]After identification of two optimal nucleophiles, we proceeded to synthesize two bifunctional reagents bearing those nucleophiles on one end and an azido group on the other. Azides 1 and 2 are both amides of 1-azido-2-aminoethane. Azide 1 has an α-hydrazino acetamido group, which is a more stable analog of the α-hydrazino acetyl group of C2H5O(O)CCH2NHNH2 (Table 1; FIG. 2); azide 2 has a γ-hydrazino acetamido group and is effectively an alkyl hydrazine.
[0113]Azide 1 was synthesized by the route in Scheme 2. Briefly, Boc-protected 1-azido-2-aminoethane was synthesized from Boc-protected 1-bromo-2-aminoethane. After Boc-deprotection, the amine was coupled to tri-Boc-protected α-hydrazino acetic acid. The Boc groups were removed, and azide 1 was isolated as a free base after cation-exchange chromatography with an overall yield of 72%.
[0114]Azide 2 was synthesized by the route in Scheme 3. Briefly, 4-pentenoic acid was subjected to ozonolysis, and t...
example 3
Kinetics of Thioester Cleavage
[0116]Kinetic studies were performed by reacting azides 1 and 2 with a model chromogenic thioester (Scheme 1).
[0117]The rate constants (k2 and k1) for azide 1 were found to be indistinguishable from those of the α-hydrazino acetyl group. The rate constants for azide 2 were, surprisingly, much lower than those of methylhydrazine. This result is contrary to our finding that methyl hydrazine is a somewhat better nucleophile than the α-hydrazino acetyl functional group (FIG. 2). The intrinsic instability of azide 2 is likely to be responsible for this apparent decrease in reactivity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chemical | aaaaa | aaaaa |
| reactivity | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com