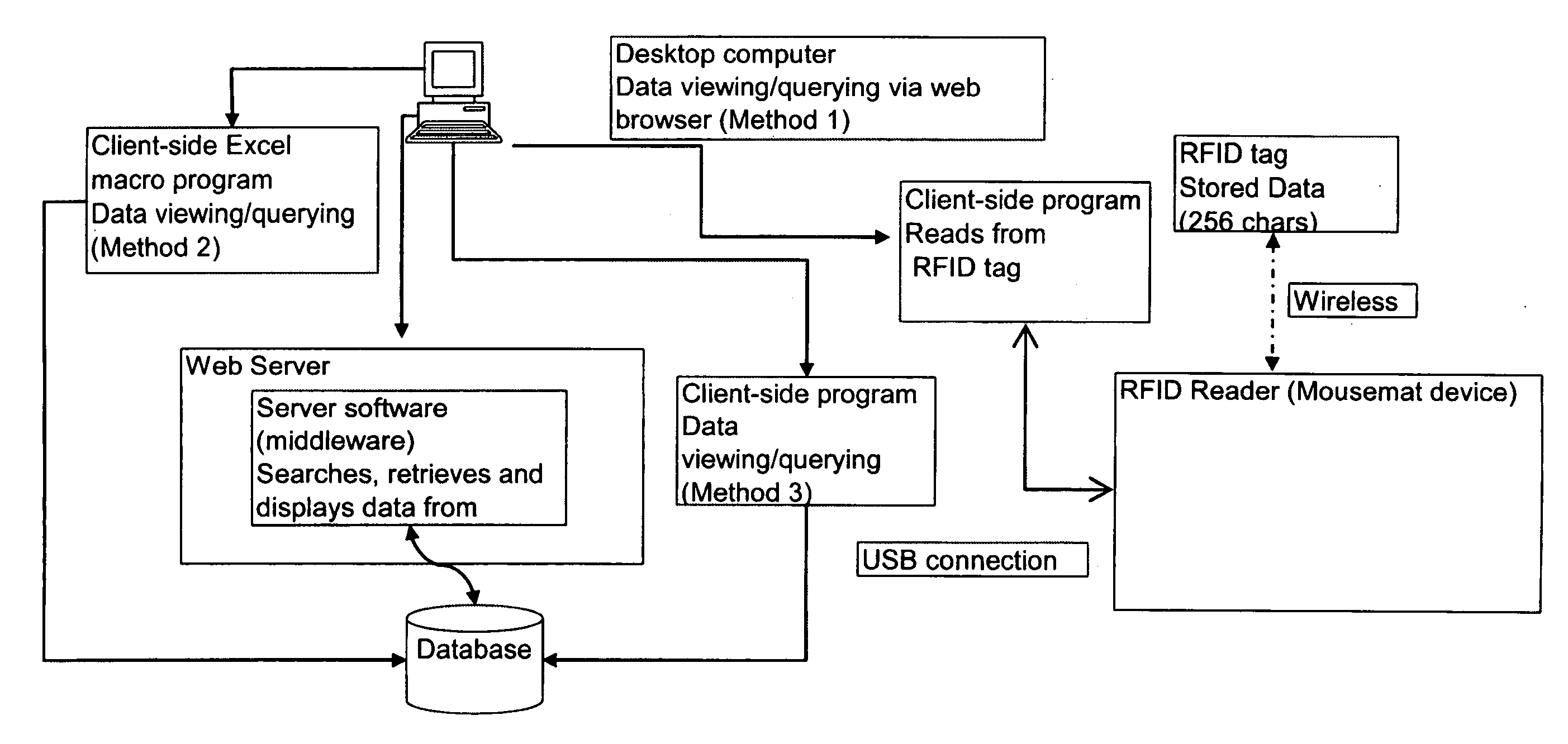
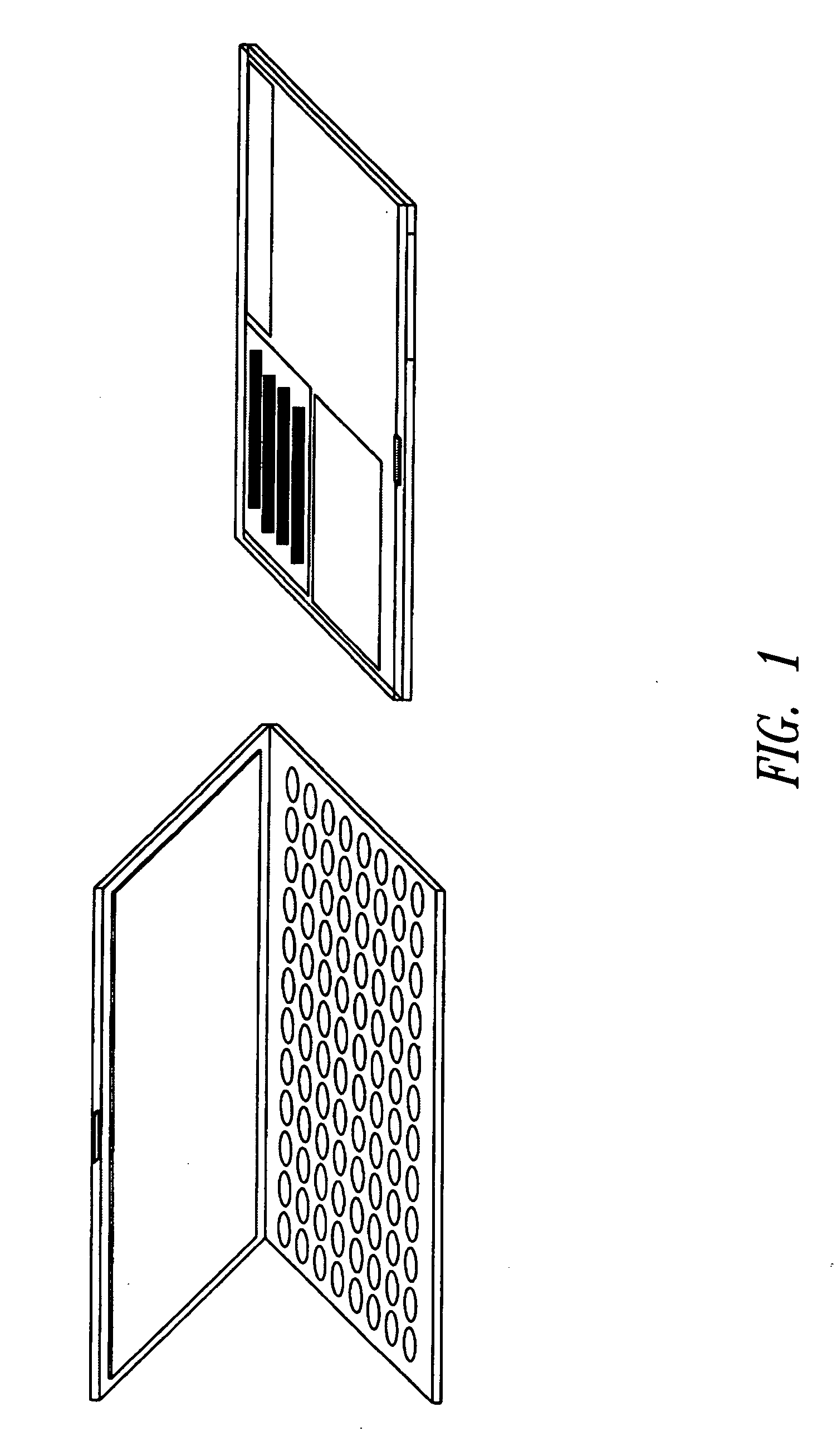
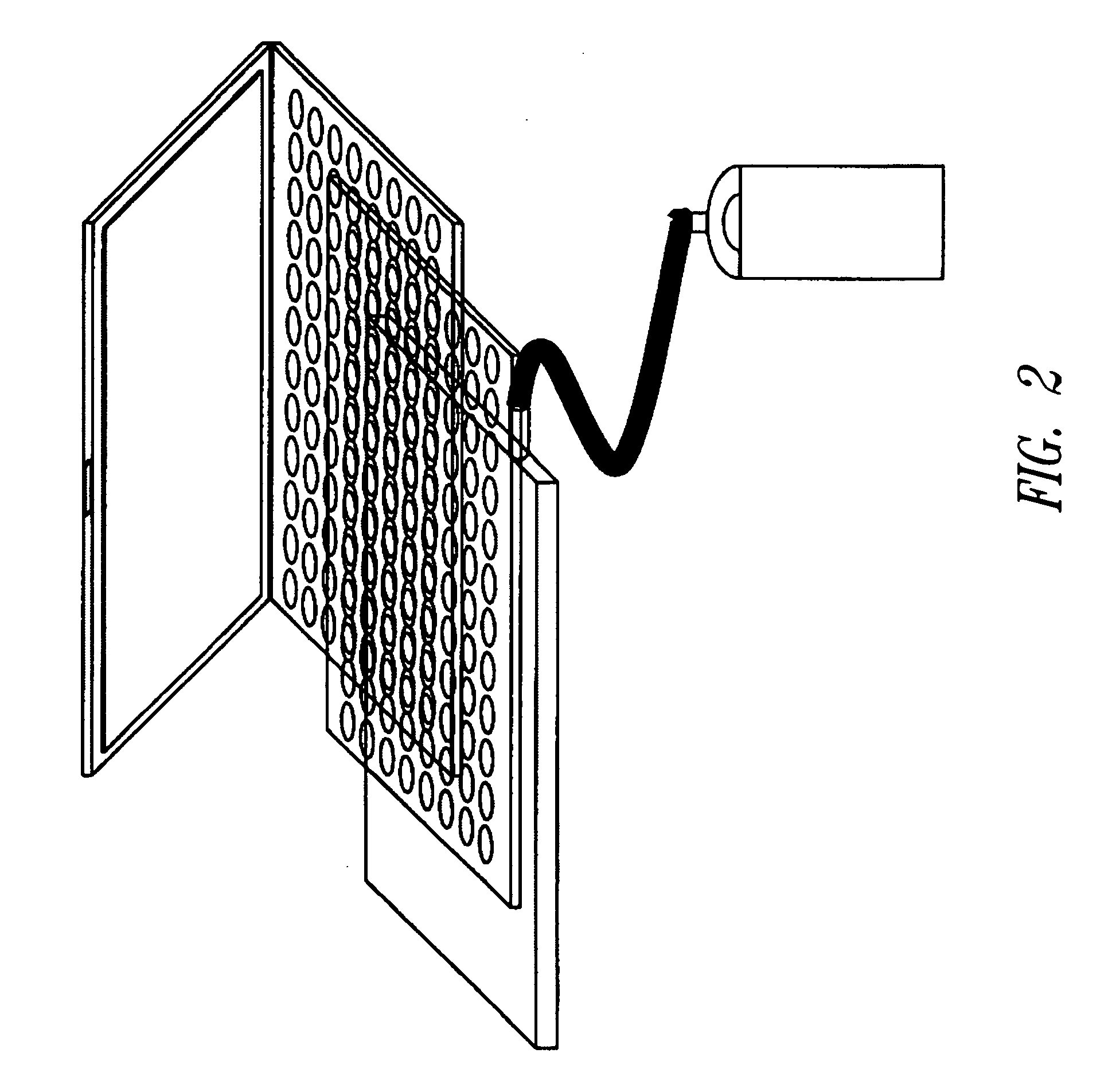
Integration of sample storage and sample management for life science
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Matrix for Biological Sample Storage Device
[0292]This example describes preparation of biological sample storage devices using a dissolvable matrix material. Dependent on the biological material being stored in a particular example, the matrix was prepared with different storage buffers. In these Examples, all reagents were from Sigma (St. Louis, Mo.) unless otherwise noted. For dry storage of nucleic acids, 20 mM Tris pH 6.5 was used for the preparation of a 1% polyvinyl alcohol (PVA, Sigma no. P8136) basic storage matrix. The concentration of the polymer was tested in a range of 0.1% to 10% (v / w). The pH of the matrix was tested in the range of pH 5 to 8. For convenient detection of biological sample phenol red was added to the liquid matrix at 0.0002% (w / v).
[0293]The matrix in liquid form was applied to sample wells of a 96-well plate and dried completely at room temperature either under standard pressure or under vacuum in a vacuum chamber. The drying time for a 5...
example 2
Dry Storage of Nucleic Acids
[0295]Biological sample storage devices were prepared as described in Example 1. General molecular biology materials and methods were used, as described. (Sambrook et al., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratories, Cold Spring Harbor, N.Y., 2001; Ausubel et al., 1993 Current Protocols in Molecular Biology, Greene Publ. Assoc. Inc. & John Wiley & Sons, Inc., Boston, Mass.). Stability tests were performed for plasmids, oligonucleotides, DNA fragments in the form of a lkB ladder, PCR products, genomic DNA (feline and human) and RNA. Recovery and stability tests were performed using gel based, PCR, and transformation rate analyses.
[0296]A. Plasmid Storage
[0297]A total of 50 ng of circular plasmid (puc19) (New England Biolabs Inc., Beverly, Mass.) at a concentration of 10 ng / μl in double distilled water (ddH2O) was spotted on the dried dissolvable matrix in each well of a 96-well polypropylene plate. The sample was dried and store...
example 3
Dry Storage of Proteins
[0314]Biological sample storage devices were prepared as described in Example 1. This example shows that dry storage of proteins at ambient temperature with complete recovery of activity offer tremendous advantages compared to storage of proteins frozen as liquid samples.
[0315]Stability and activity tests for different sequenases, heat stable polymerases, restriction enzymes, ligases, proteases were performed to demonstrate the protective nature of the dissolvable matrix. Stabilization of proteins and their recovery as active molecules was achieved using the longterm dissolvable matrix described above. The matrix was prepared in the presence of TRIS pH5-8, phenol red as a pH indicator, and 1% PVA. The matrix was solidified by dehydration and the proteins were spotted onto the dried matrix in the presence or absence of trehalose (Fluka, cat. no. 90210) or validamycin A (Research Products International Corp., catalog no. V21020) in liquid form. The water in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com