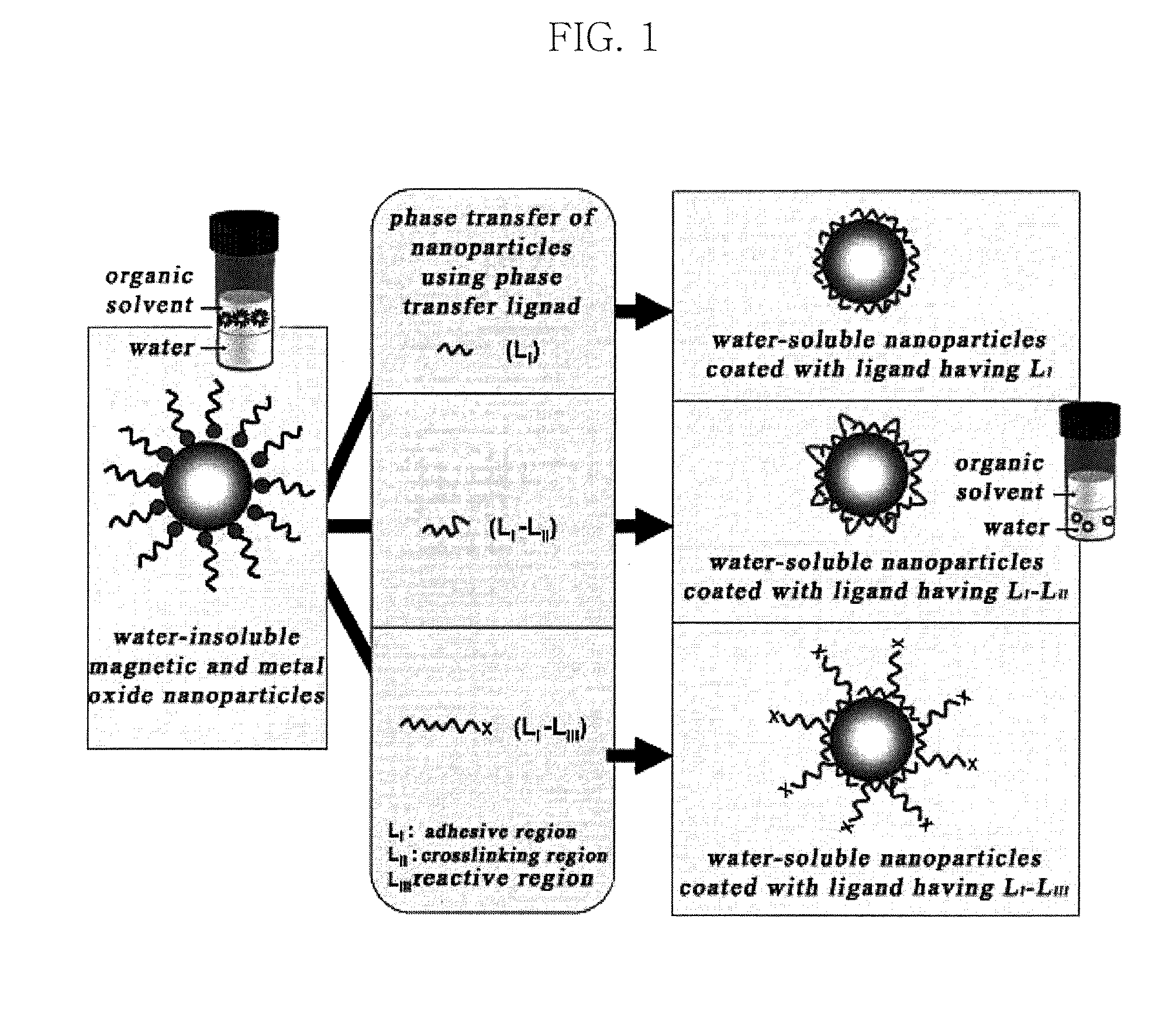
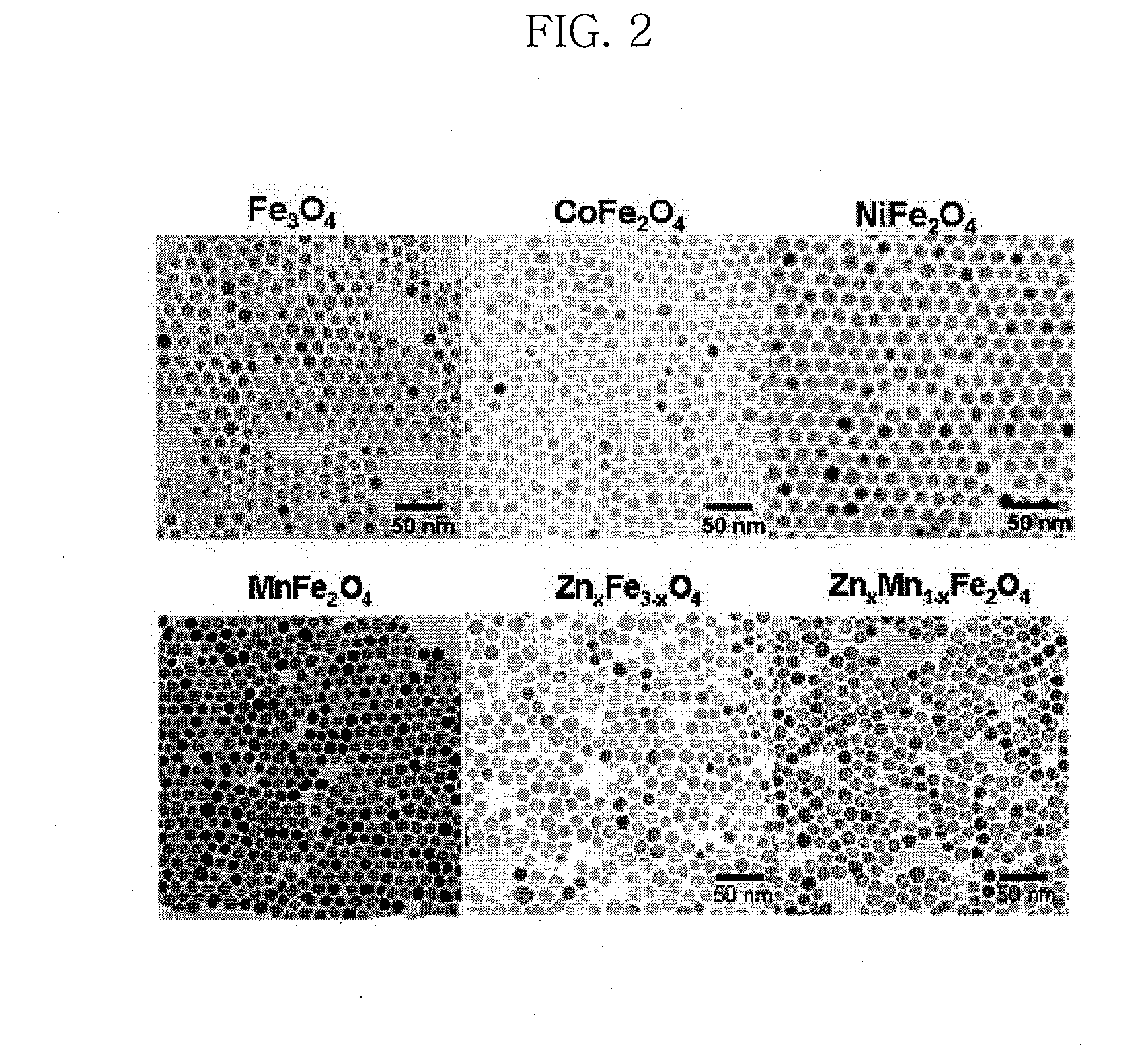
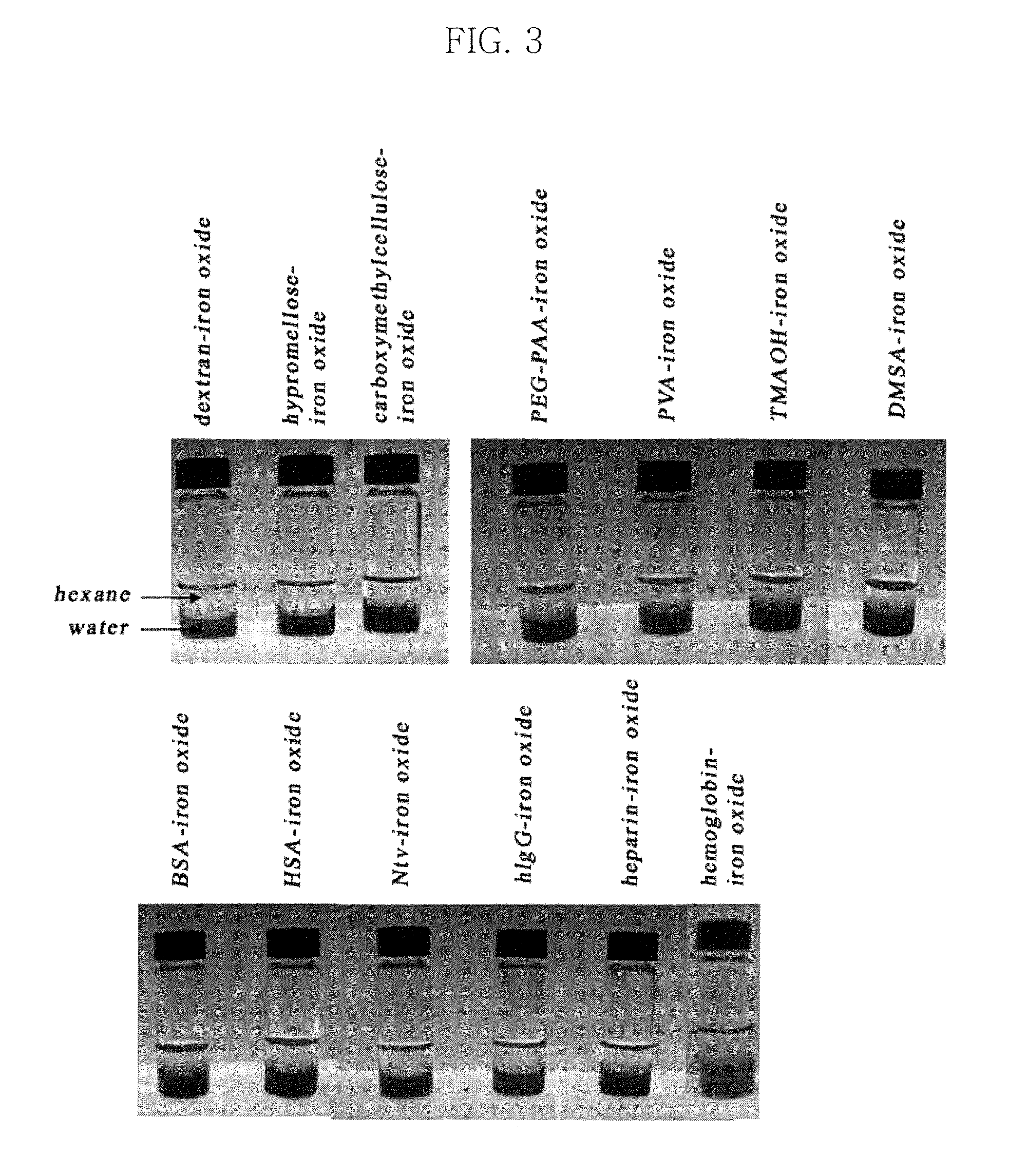
Water-soluble magnetic or metal oxide nanoparticles coated with ligands, preparation method and usage thereof
a technology of magnetic or metal oxide nanoparticles and ligands, applied in the direction of granular delivery, powder delivery, energy modified materials, etc., can solve the problems of difficult control of the size of the nanoparticle, low crystallinity of the nanoparticle, and non-stoichiometric compound, etc., and achieve the effect of stably dissolving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Water-Soluble Iron Oxide, Manganese Ferrite, Cobalt Ferrite, Nickel Ferrite, Zinc Ferrite, and Zinc-Manganese Ferrite. Nanoparticles Coated with Bovine Serum Albumin (BSA, LI-LIII)
[0115]Water-insoluble iron oxide, manganese ferrite, cobalt ferrite, nickel ferrite, zinc ferrite, and zinc-manganese ferrite nanoparticles were synthesized according to the technique disclosed in Korean Patent Application Nos. 2004-0070303 and 2004-0070304, filed by the present inventors. All of the obtained nanoparticles were observed to have a particle size of about 12 nm, as intended, and to have a uniform size distribution and high crystallinity (FIG. 2).
[0116]The water-insoluble nanoparticles (5 mg) were dispersed in 1 ml of 1 M NMe4OH butanol solution and were then mixed for about 5 minutes, until uniform. Thereafter, a blackish brown precipitate was formed, and this precipitate was separated through centrifugation (2000 rpm, room temperature, 5 min). 10 mg of BSA was dissolved in 1 ml ...
example 2
Synthesis of Water-Soluble Iron Oxide, Manganese Ferrite, Cobalt Ferrite, Nickel Ferrite, Zinc Ferrite, and Zinc-Manganese Ferrite Nanoparticles Coated with Human Serum Albumin (HSA, LI-LIII)
[0117]As water-insoluble iron oxide, manganese ferrite, cobalt ferrite, nickel ferrite, zinc ferrite, and zinc-manganese ferrite nanoparticles, the same iron oxide, manganese ferrite, cobalt ferrite, nickel ferrite, zinc ferrite, and zinc-manganese ferrite nanoparticles as those of Example 1 were used. The water-insoluble nanoparticles (5 mg) were dispersed in 1 ml of 1 M NMe4OH butanol solution and were then mixed for about 5 minutes, until uniform. Thereafter, a blackish brown precipitate was formed, and this precipitate was separated through centrifugation (2000 rpm, room temperature, 5 min). 10 mg of HSA was dissolved in 1 ml of deionized water and was then mixed with the above precipitate, thus synthesizing iron oxide, manganese ferrite, cobalt ferrite, nickel ferrite, zinc ferrite, and zin...
example 3
Synthesis of Water-Soluble Iron Oxide, Manganese Ferrite, Cobalt Ferrite, Nickel Ferrite, Zinc Ferrite, and Zinc-Manganese Ferrite Nanoparticles Coated with Human ImmunoGlobulin G (hIgG, LI-LIII)
[0118]As water-insoluble iron oxide, manganese ferrite, cobalt ferrite, nickel ferrite, zinc ferrite, and zinc-manganese ferrite nanoparticles, the same iron oxide, manganese ferrite, cobalt ferrite, nickel ferrite, zinc ferrite, and zinc-manganese ferrite nanoparticles as those of Example 1 were used. The water-insoluble nanoparticles (5 mg) were dispersed in 1 ml of 1 M NMe4OH butanol solution and were then mixed for about 5 minutes, until uniform. Thereafter, a blackish brown precipitate was formed, and this precipitate was separated through centrifugation (2000 rpm, room temperature, 5 min). 10 mg of hIgG was dissolved in 1 ml of deionized water and was then mixed with the above precipitate, thus synthesizing iron oxide, manganese ferrite, cobalt ferrite, nickel ferrite, zinc ferrite, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com