Method of producing biodiesel fuel
a biodiesel and fuel technology, applied in biofuels, biofeedstocks, fuels, etc., can solve the problems of insufficient conversion ratio in other methods, inability to meet the requirements of biodiesel, and the foregoing refining process may decompose biodiesel itself, etc., to achieve high viscosity, easy removal, and high viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
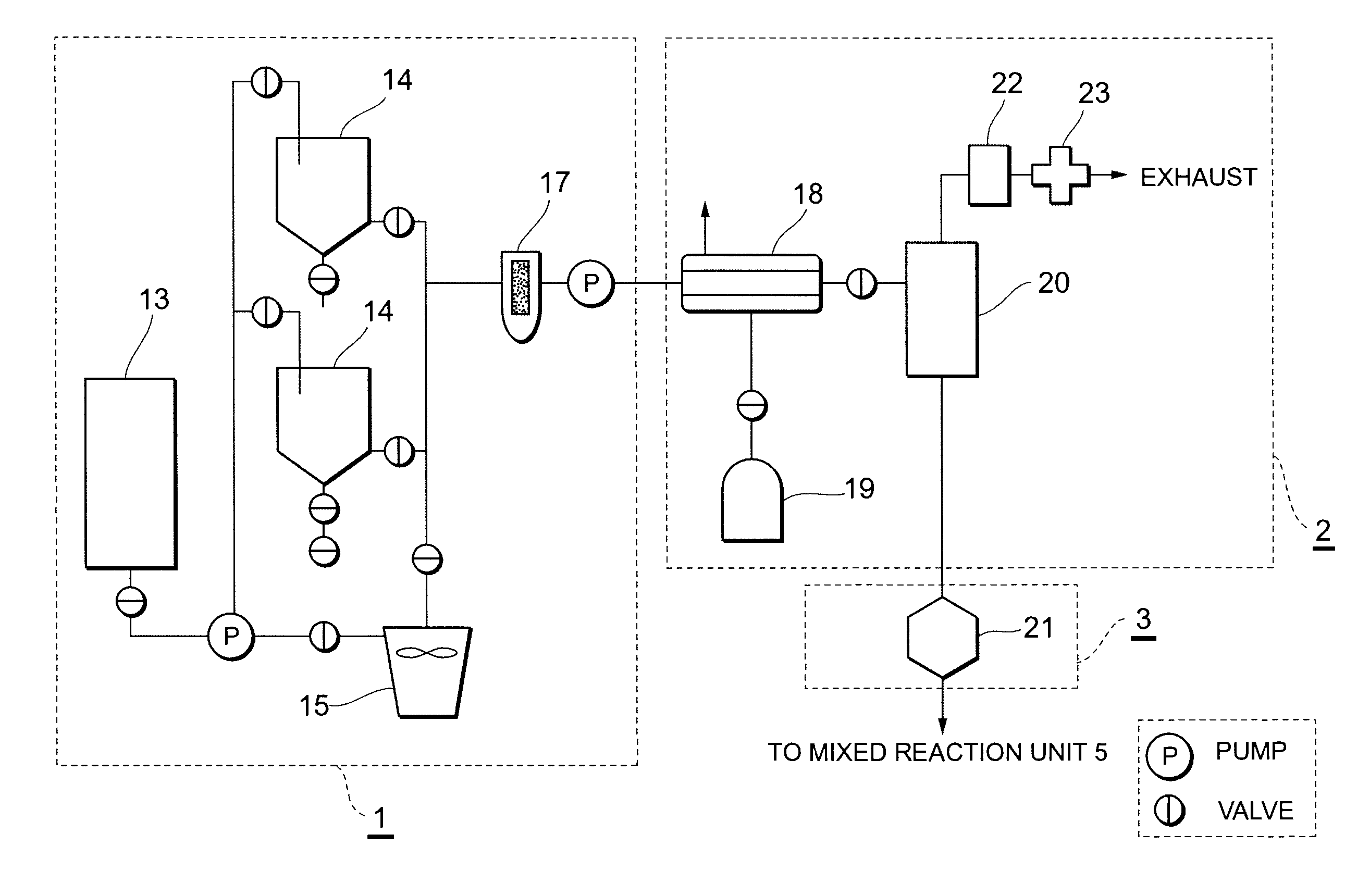
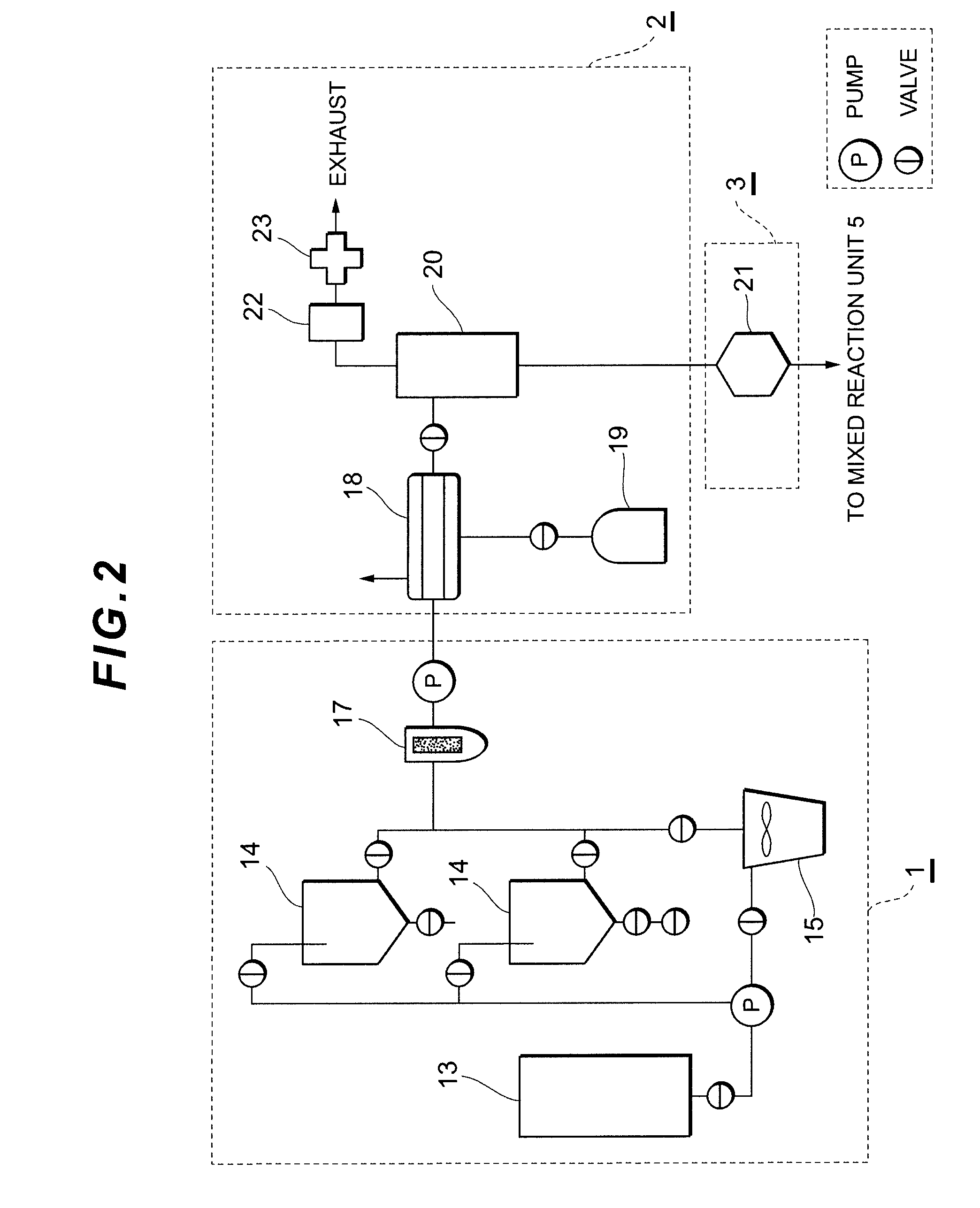
[0093]In the production apparatus configured as shown in FIG. 1 to FIG. 5 and having a processing / production capacity of 20 tons / day, a stainless net of 120 mesh was used as the filter to be mounted at the reception opening of the raw material oil reception tank 13. A polyester continuous fiber filter of 300 mesh was used as the filter to be mounted on the strainer 17 to be mounted on the exit side of the raw material oil storage tank 14 or the solid-liquid, liquid-liquid centrifugal separator 15.
[0094]Spent waste vegetable oil (acid value: 5.2, iodine number: 108, flash point 230° C., moisture 1.1%) that was used in restaurants and the like was received in the raw material oil reception tank 13, and then fed into the raw material oil storage tank 14. After causing the raw material oil to naturally deposit for 8 hours, the supernatant was used in the subsequent step onward. Potassium hydroxide (purity 90%) was used as the catalyst, and the catalyst dissolution bath 27 was used to di...
example 2
[0105]Other than using rapeseed oil (acid value: 0.6, iodine number: 118, flash point 230° C., moisture 0.2%) as the raw material oil, the method was performed as with Example 1. The results of the property analysis are shown in Table 1.
example 3
[0106]Other than using soybean oil (acid value: 0.6, iodine number: 132, flash point 240° C., moisture 0.2%) as the raw material oil, the method was performed as with Example 1. The results of the property analysis are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pour point | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| vapor pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com