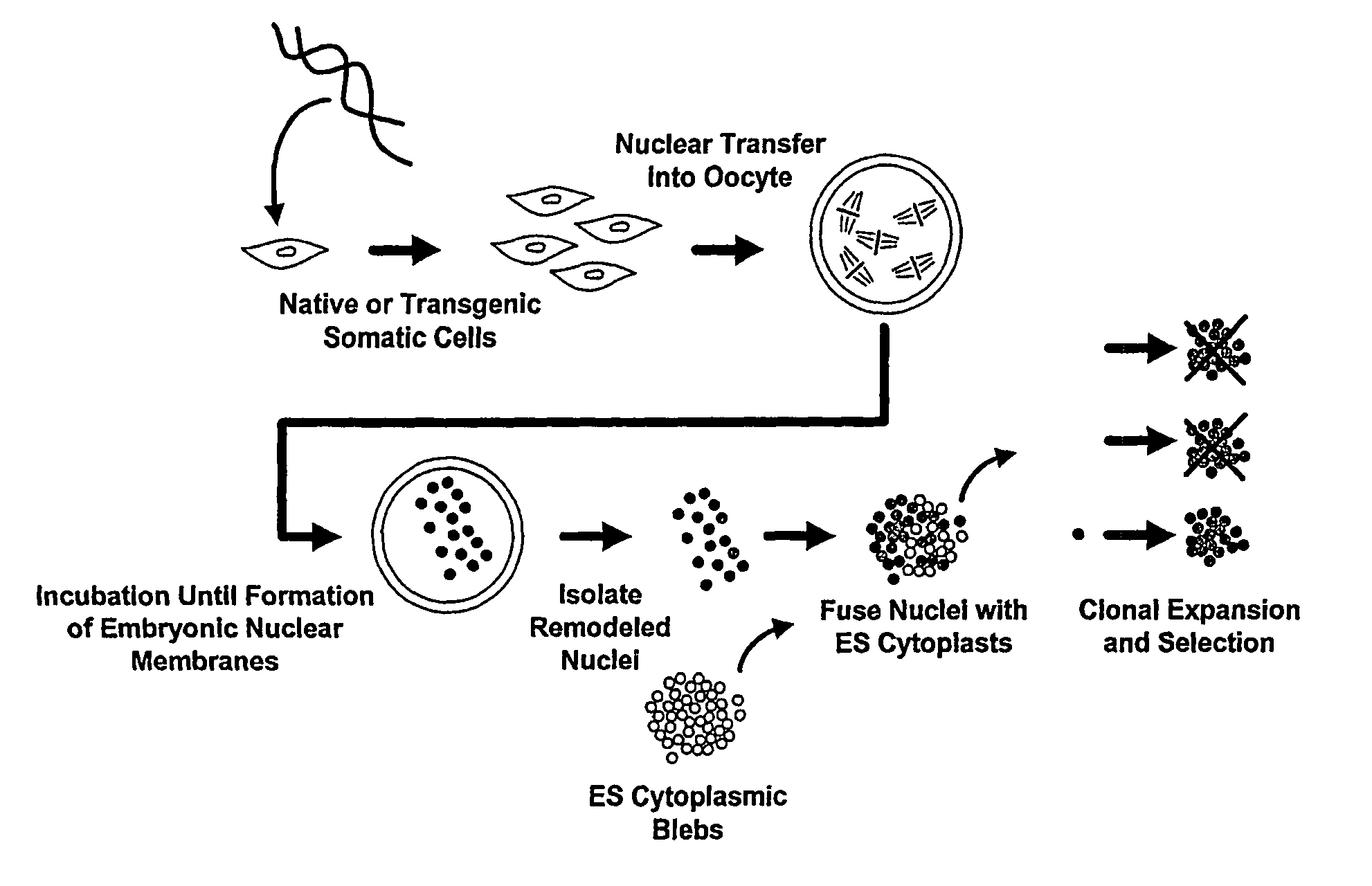
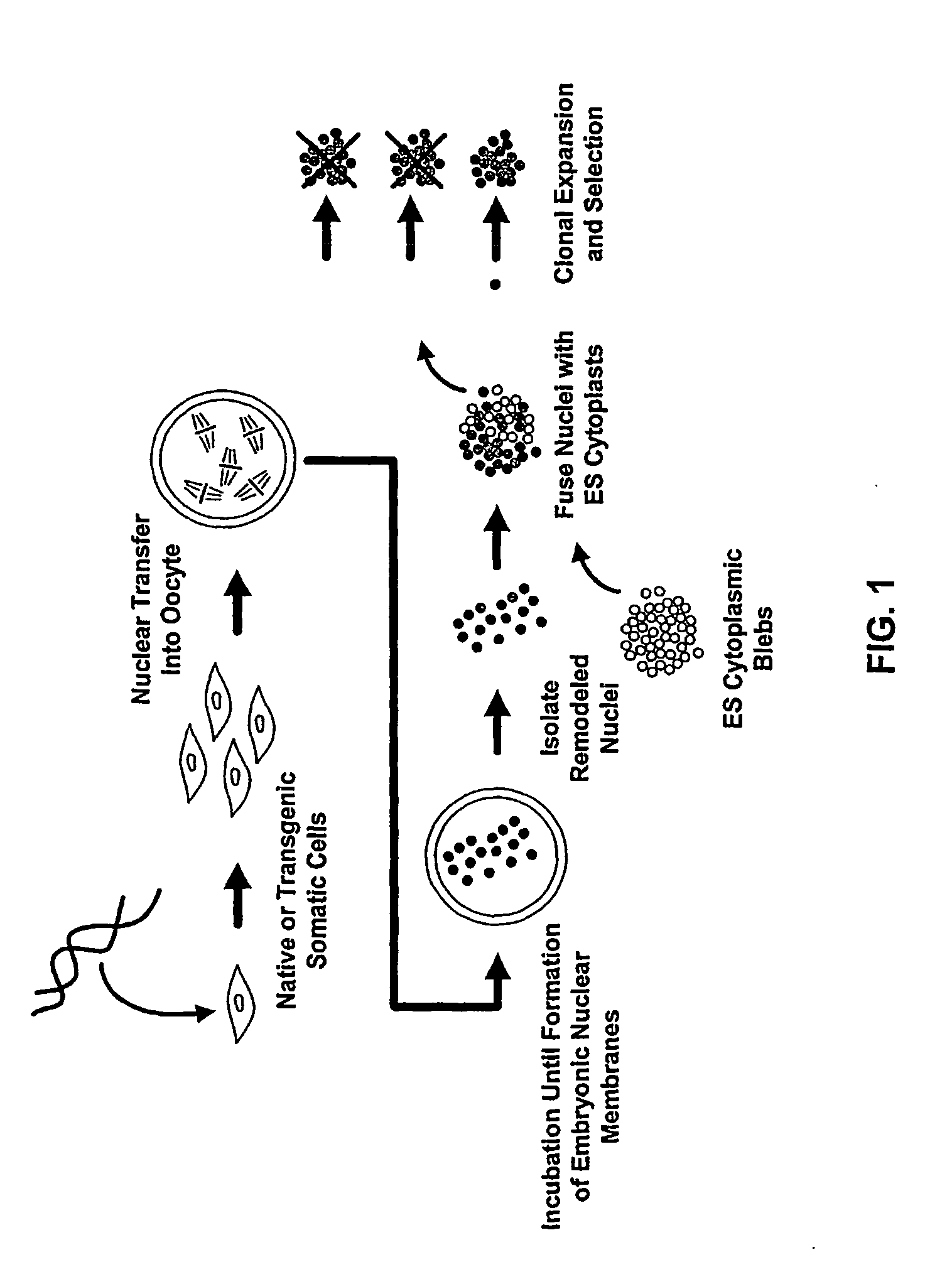
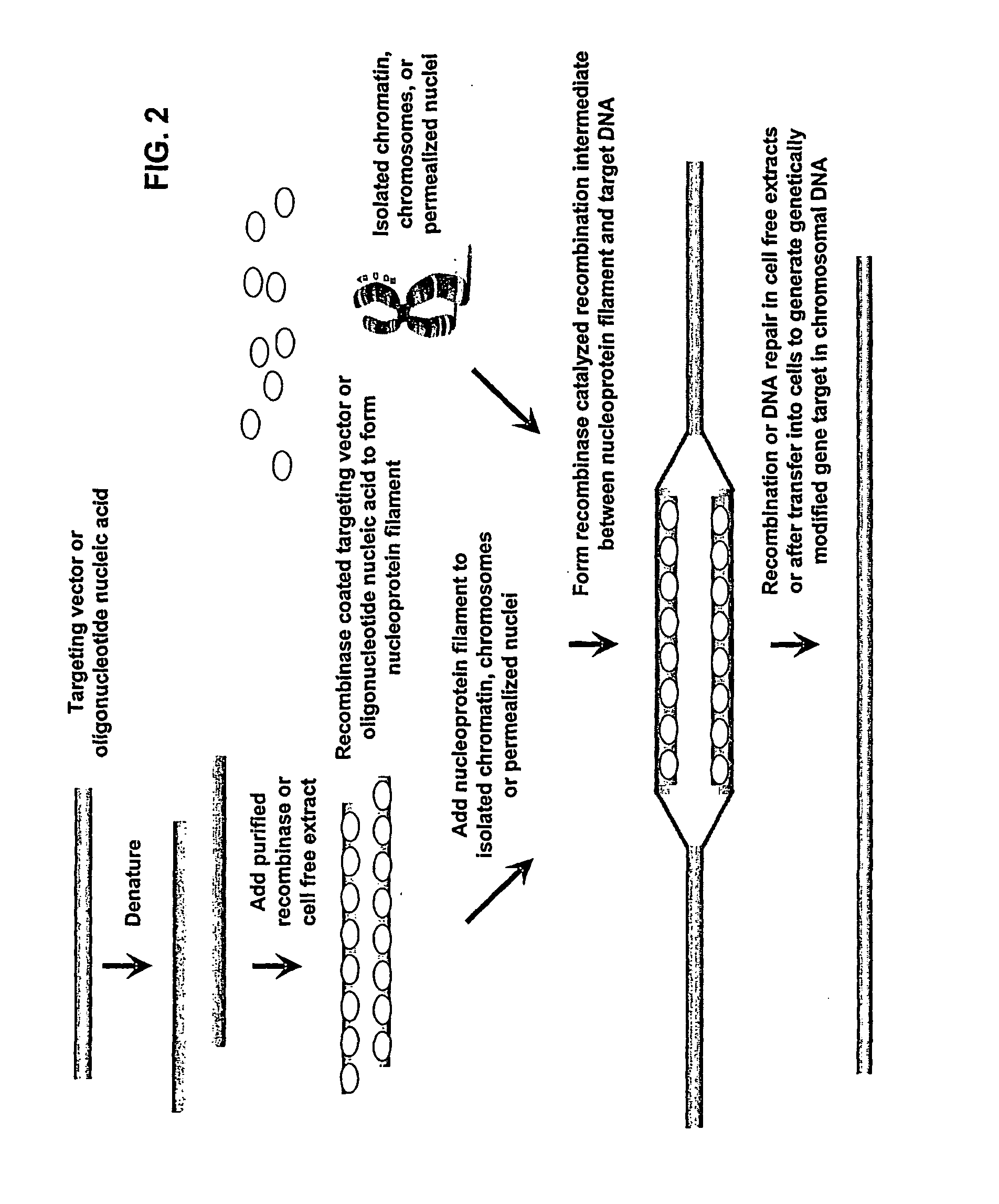
Methods of Reprogramming Animal Somatic Cells
a somatic cell and reprogramming technology, applied in the field of reprogramming an animal somatic cell, can solve the problems of inefficiency or incomplete reprogramming of cells using existing technologies, inability to adapt to normal or histocompatibility cells for transplantation, and inability to use cross-species nuclear transfer, etc., to achieve the effect of replicative lifespan and extension of telomere length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Nuclear Remodeling
[0129]The first step (also referred to herein as the “nuclear reprogramming step”) is performed using human peripheral blood mononuclear cells which are purified from blood using Ficoll gradient centrifugation to yield a buffy coat comprised primarily of lymphocytes and monocytes as is well known in the art. The use of lymphocytes with a rearranged immunoglobulin locus as donors in the present invention will result in stem cells with the same rearranged loci. In the case where the desired outcome of the experiment is not cells with a preformed rearrangement in immunoglobulin genes, the monocytes are purified from the lymphocytes by flow cytometry as is well known in the art and stored at room temperature in Dulbecco's minimal essential medium (DMEM) or cryopreserved until use. Xenopus oocytes from MS222 anesthetized mature females are surgically removed in MBS buffer and inspected for quality as is well-known in the art (Gurdon, Methods Cell Biol 16:125-139, (1977)...
example 2
Nuclear Remodeling
[0130]In this example, step one of nuclear remodeling is carried out in an extract from undifferentiated cells of the same species as the differentiated cell; human dermal fibroblasts nuclei are remodeled in vitro using mitotic cell extracts from the human embryonal carcinoma cell line NTera-2. However, extracts from cells of a different species may alternatively be used.
Preparation of Nuclear Remodeling Extract
[0131]NTera-2 cl. D1 cells are easily obtained from sources such as the American Type Culture Collection (CRL-1973) and are grown at 37° C. in monolayer culture in DMEM with 4 mM L-glutamine, 1.5 g / L sodium bicarbonate and 4.5 g / L glucose, 10% fetal bovine serum (complete medium). While in a log growth state, the cells are plated at 5×106 cells per sq cm tissue culture flask in 200 mL of complete medium. Extracts from cells in the prometaphase are prepared as is known in the art (Burke & Gerace, Cell 44: 639-652, (1986)). Briefly, after two days and while st...
example 3
Genetic Modification of Remodeled Nuclei or Chromatin
[0137]The isolated nuclei or condensed chromatin may optionally be modified by methods involving recombinase treated targeting vectors or oligonucleotides. The DNA from cell free chromosomes and chromatin can be genetically modified enzymatically with targeting vectors or oligonucleotides, using purified recombinases, purified DNA repair proteins, or protein or cell extract preparations comprising such proteins. The targeting DNAs may have tens of kilobasepairs to oligonucleotides of at least 50 basepairs of homology to the chromosomal target. Recombinase catalyzed recombination intermediates formed between target chromosomes and vector DNA can be enzymatically resolved in cell free extracts with other purified recombination or DNA repair proteins to produce genetically modified chromosomes. These modified chromosomes can be reintroduced into cells or used in the formation of nuclei in vitro prior to introduction into cells; modif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gene expression profile | aaaaa | aaaaa |
| Recombination enthalpy | aaaaa | aaaaa |
| Exposure limit | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com