Compositions and methods for biological sample storage
a biological sample and composition technology, applied in the field of compositions and methods for biological sample storage, can solve the problems of inconvenient maintenance of adequate low temperature, unreliable equipment, and disadvantages of both storage systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Matrix for Biological Sample Storage
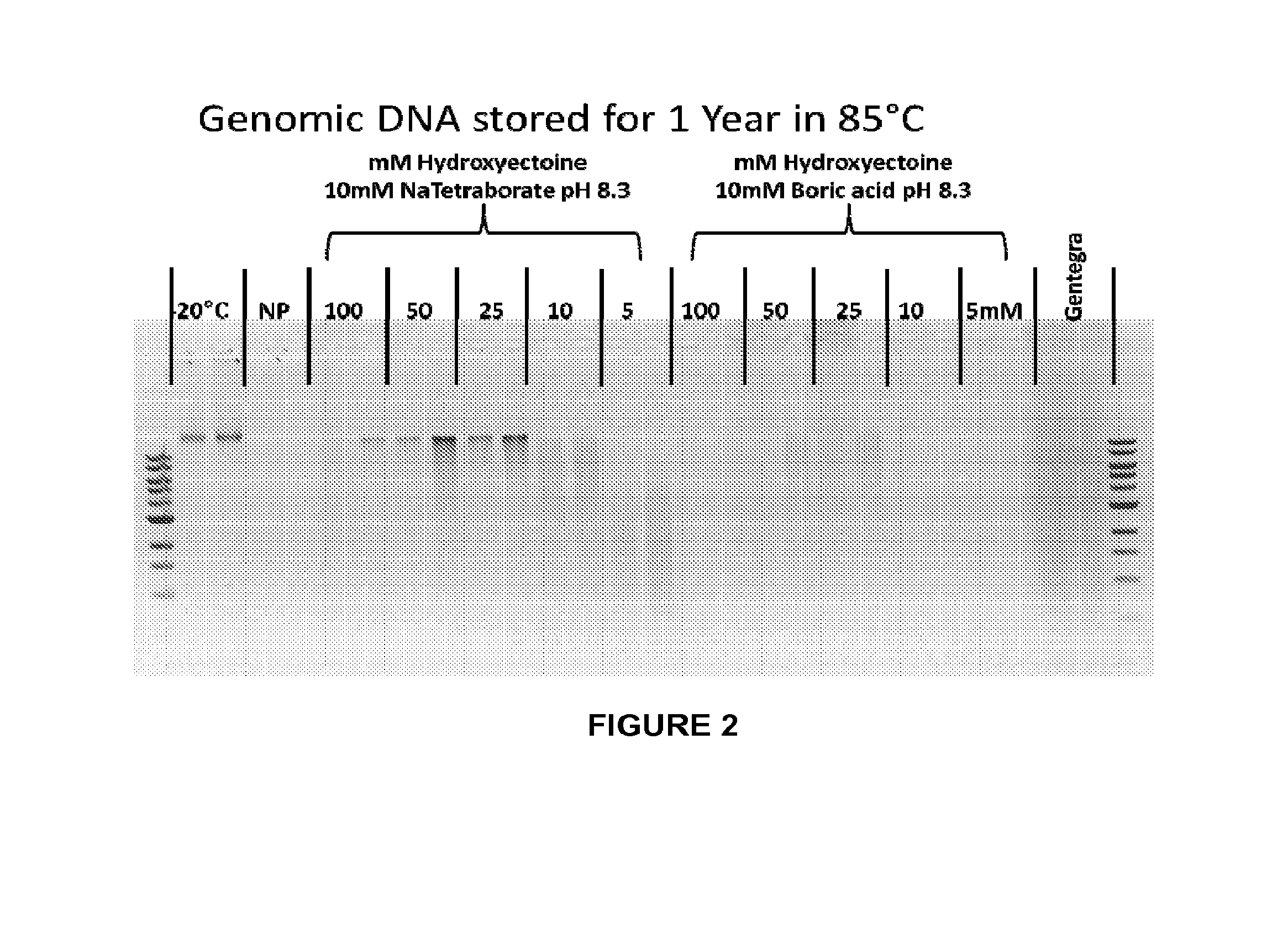
[0189]This example describes preparation of a dissolvable matrix comprising a borate composition and a stabilizer as provided herein, for storage of biological sample material, including unrefrigerated substantially dry storage. Unless otherwise noted, all reagents to which reference is made in these Examples were from Sigma-Aldrich (St. Louis, Mo.). 191 mg of sodium tetraborate decahydrate was placed in a 50 mL conical tube and then 158 mg of hydroxyectoine was added. RNase- and DNase-free 18.2 megaOhm water was added to bring the total volume to 50 mL. The mixture was stirred until the solids were completely dissolved.
[0190]The matrix in liquid form was applied to sample wells of a 96-well plate and dried completely at room temperature either under standard pressure or under vacuum in a vacuum chamber. The drying time for a 20-50 μl volume of the borate-stabilizer matrix was overnight, and under vacuum a shorter drying time was re...
example 2
Dry Storage of Nucleic Acids
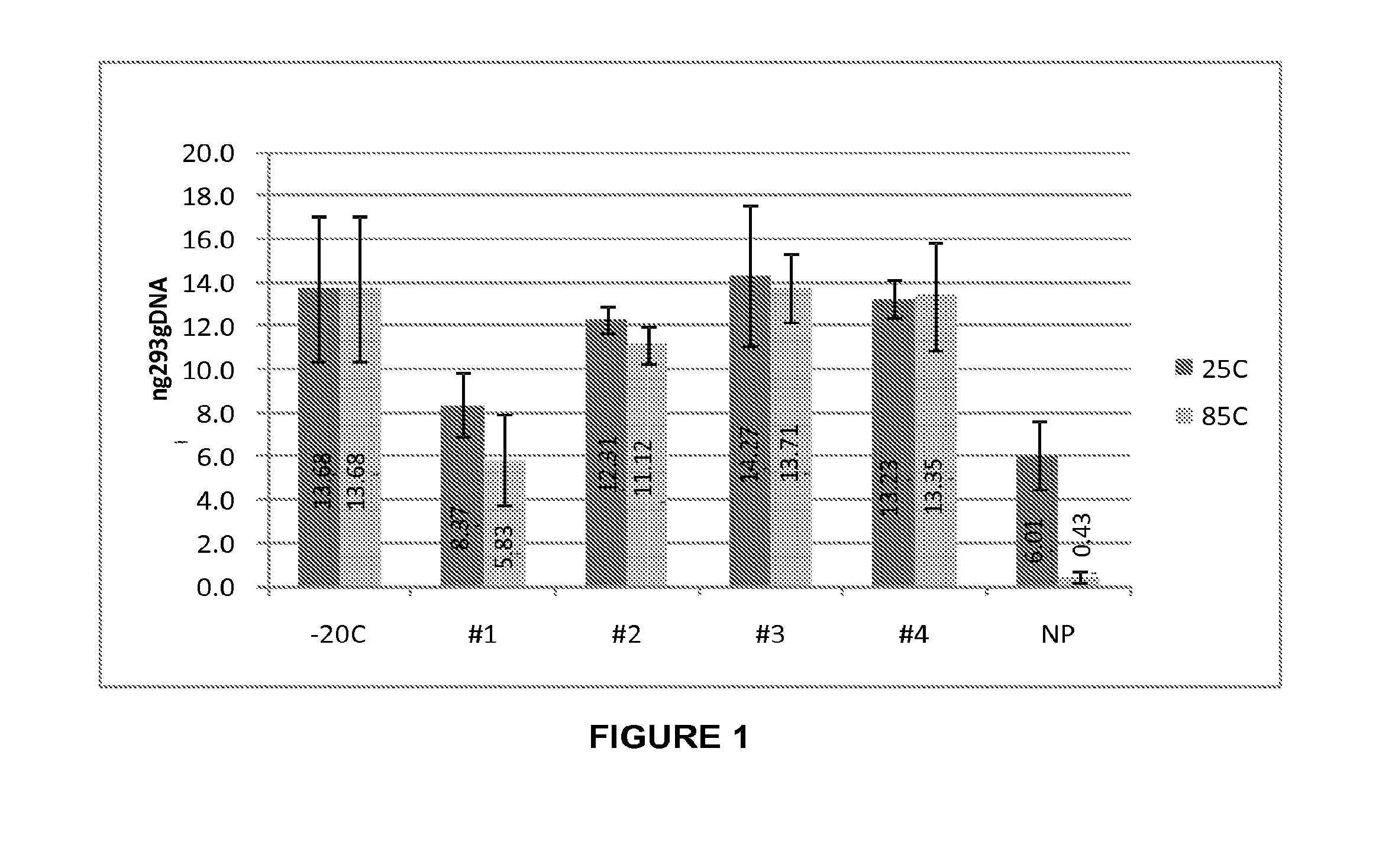
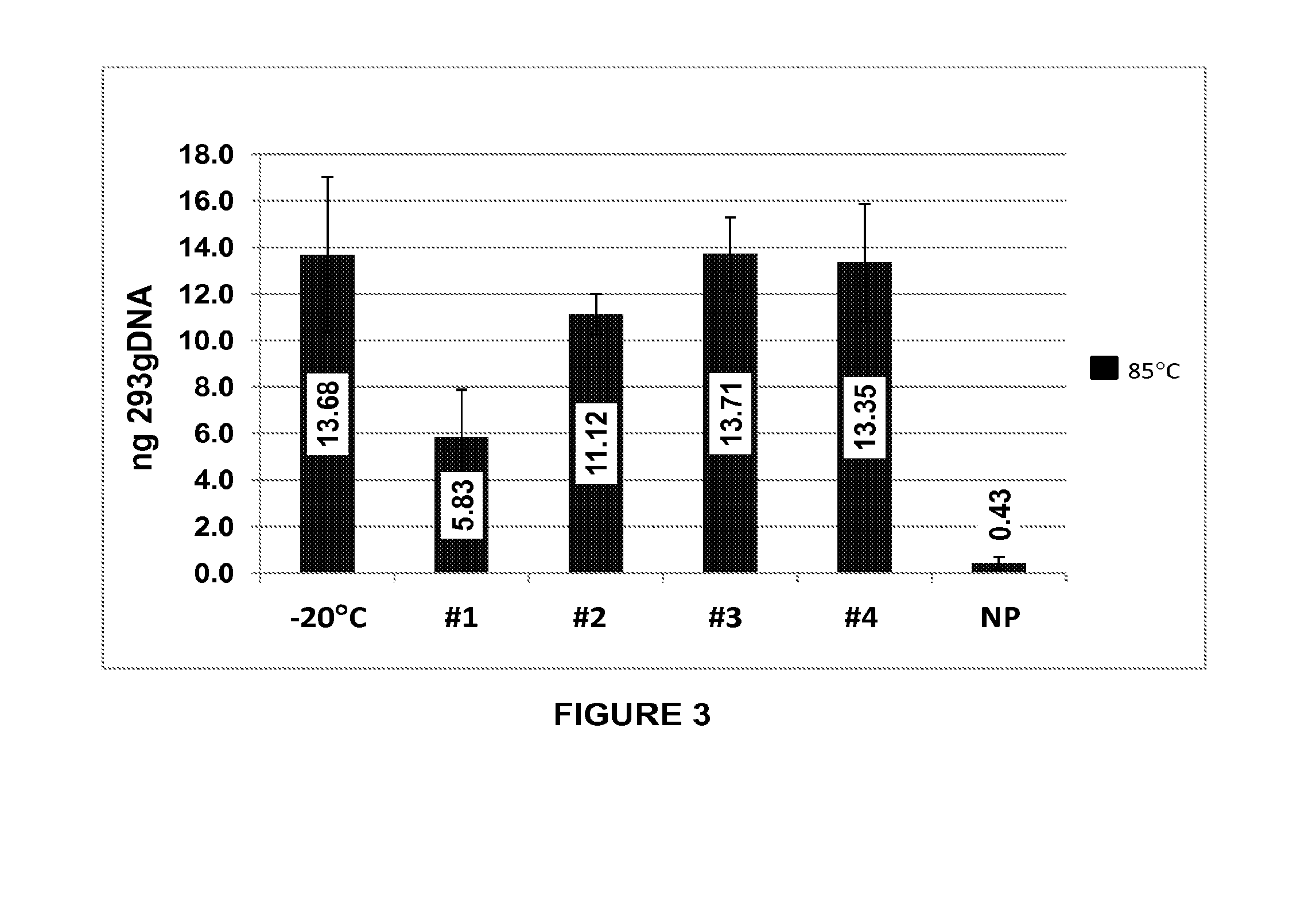
[0192]Biological sample storage devices were prepared with dried borate / hydroxyectoine matrices as described in Example 1. General molecular biology materials and methods were used, as described. (Sambrook et al., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratories, Cold Spring Harbor, N.Y., 2001; Ausubel et al., 1993 Current Protocols in Molecular Biology, Greene Publ. Assoc. Inc. & John Wiley & Sons, Inc., Boston, Mass.). Stability tests were performed for plasmids, oligonucleotides, DNA fragments in the form of a 1 kB ladder, PCR products, genomic DNA (feline and human) and RNA. Recovery and stability tests were performed using electrophoretic gel-based, polymerase chain reaction (PCR), and transformation rate analyses.
A. Genomic Human DNA
[0193]A total of 100 ng of human genomic DNA (Novagen / EMD4 Biosciences, San Diego, Calif.) in Tris-EDTA (TE) buffer-pH 8 was applied directly into wells of a 96-well plate that either contained the...
example 3
Storage of Human Genomic DNA for Three Months at 85° C.
[0195]Human genomic DNA samples prepared as described in Example 2 were analyzed after 90 days of dry storage at 85° C. Samples were rehydrated with sample loading buffer and applied to an 0.8% agarose gel and electrophoresed at a constant 150 V for 40 min. Gel images were obtained using a KODAK-100 gel imager (Eastman Kodak, Rochester, N.Y.) using an ethidium bromide filter and excitation of the DNA bands with 302 nm UV light with exposure set at 0.15 seconds. Highly intact DNA was recovered from borate / stabilizer matrix-containing wells and could be observed migrating in gel electrophoresis with an apparent molecular mass greater than the 23 kb fragment of the reference standard DNA ladder for the samples with which the borate / stabilizer matrix was used to protect the DNA. Unprotected DNA (i.e., DNA that had been dry-stored in wells in the absence of any matrix) was degraded to the point where it could not be visualized by thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com