Collagen scaffold modified by covalent grafting of adhesion molecules, associated methods and use thereof for cardiovascular and thoracic cell therapy and contractile tissue engineering
a collagen scaffold and covalent grafting technology, applied in the field of collagen scaffolds, can solve the problems of lack of differentiation of transplanted cells, difficult production of cell therapy scaffolds, and substantial cell mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
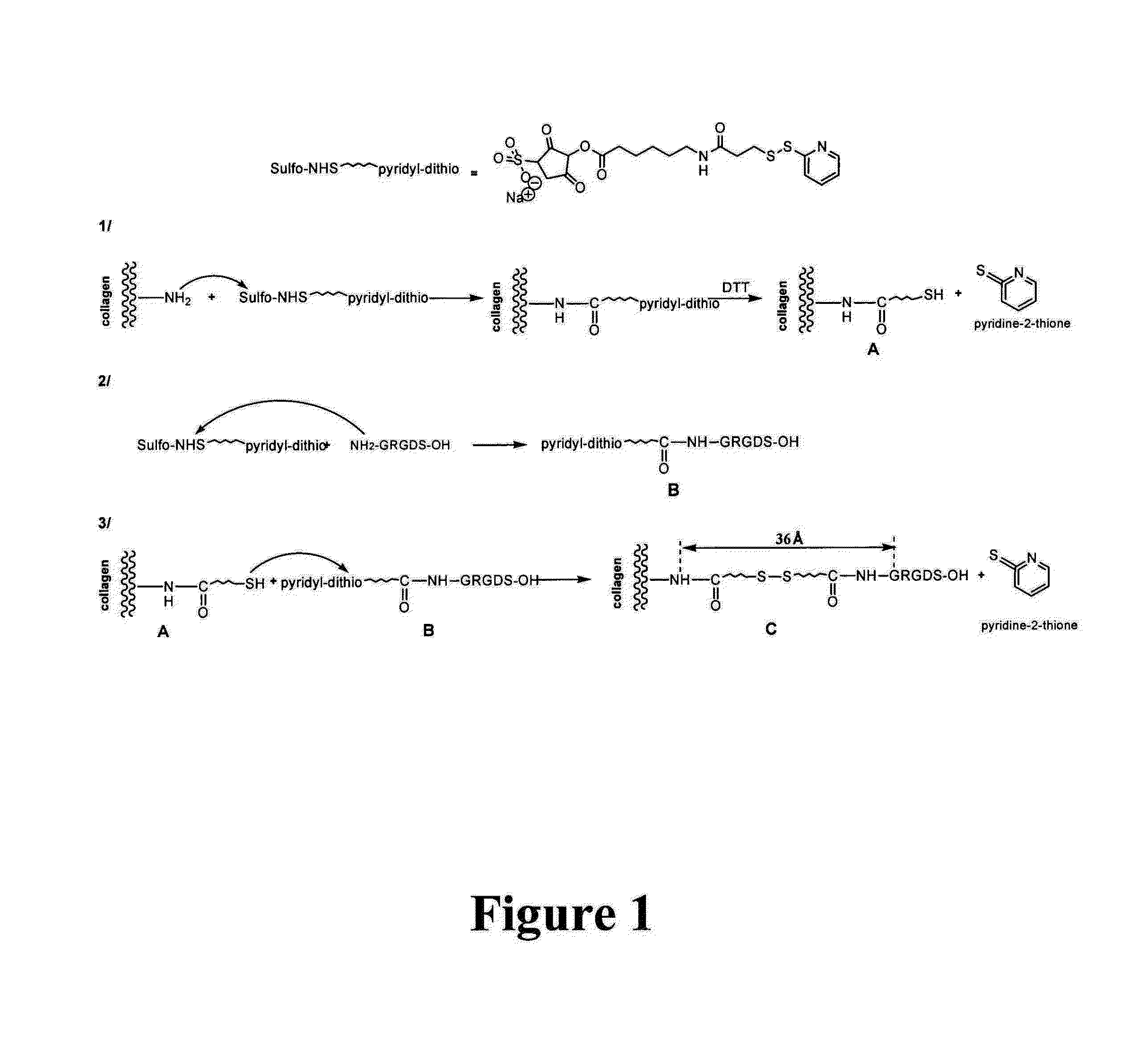
[0085]Covalent coupling of adhesion molecules such as RGD / RGE peptides to the collagen scaffold or biological agents such as proteoglycans, growth factors or cytokines if the scaffold is made of collagen and / or contains thiol, amine or carboxyl accessible groups.
[0086]The coupling chemical reaction principle is illustrated in FIG. 1. The coupling described here makes it possible to illustrate the RGD moiety at a medium distance from the support, here a 30-40 Angstrom collagen matrix, elongation that is favorable to the maximum interaction of the peptide sequence with the integrin receptor site (cf. Beer J H. and al. 1992, Craig W S. and al. 1995)23,24.
[0087]Collagen scaffolds are used, and advantageously DHT-cross-linked porous supports like with ULTRAFOAM®, materials that are already used in clinical medicine as hemostatics: 5 mm-thick ULTRAFOAM® sheets (2.5 mm after rehydration in PBS) (Davol Inc., Cranston, R.I.) come from beef, DHT-cross-linked type I and type III collagen fiber...
example 2
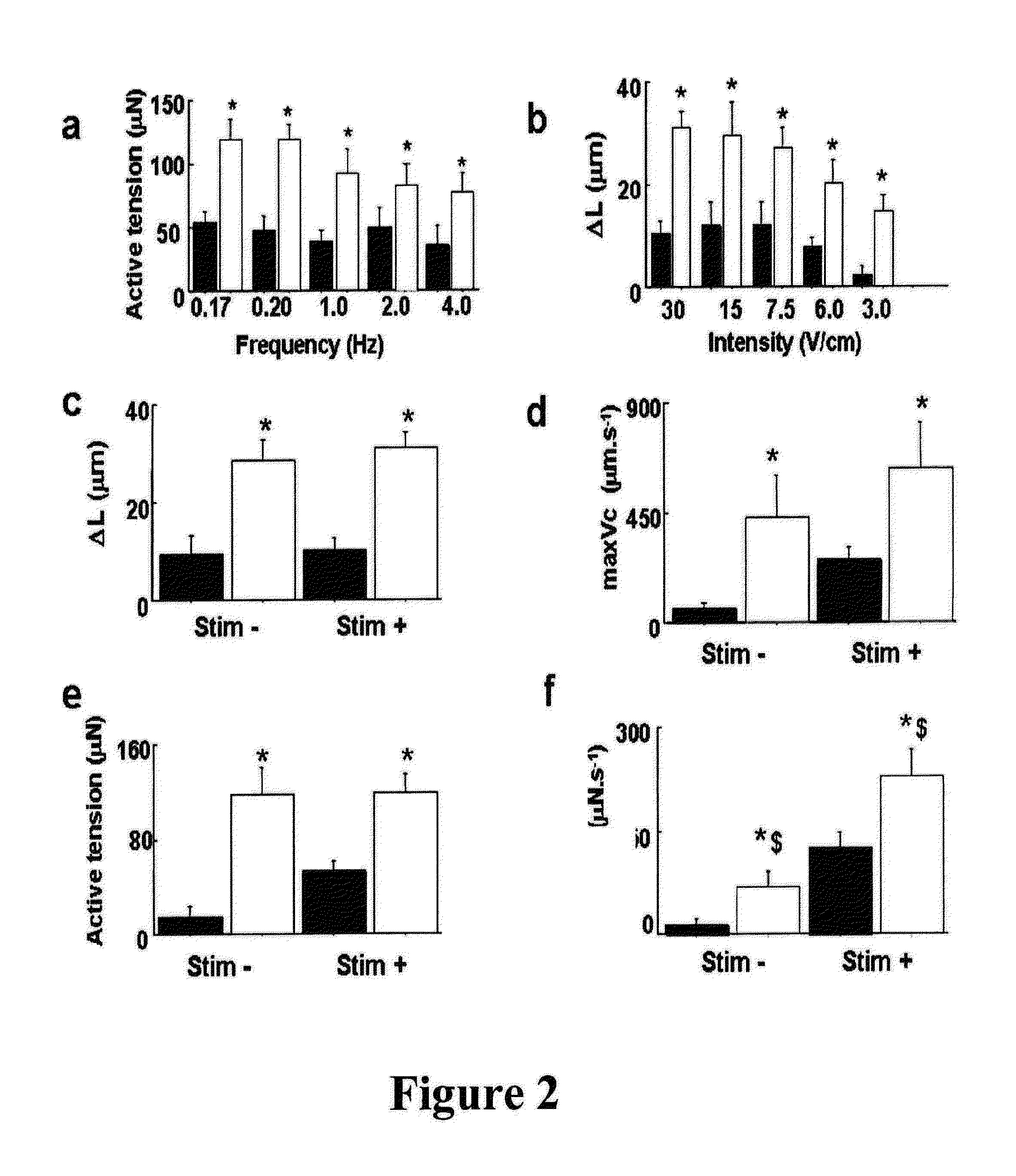
[0089]Using collagen scaffolds functionalized with adhesion molecules for making a contractile tissue
[0090]Contractile Cells
[0091]Different cell types have a contractile activity prior to or after a complete differentiation as for example smooth muscle cells, skeletal muscle cells or cardiac myocytes. Embryonic stem cells, pre-differentiated or not (where the differentiating agent may be for example a growth factor or a combination of growth factors. The removal or the inhibition of some factors from the culture medium as for example growth factors of the FGF, TGF beta, BMP-2, SDF1 type, physical factors such as hypoxia, electrostimulation, freezing, mechanical stress, etc.) also has shown their ability to promote the differentiation towards contractile cells. Marrow bone cells (hematopoietic cells or mesenchymal cells), cells isolated from the circulating blood (also including cells isolated from the umbilical cord blood). Contractile potential cells were also isolated from differe...
example 3
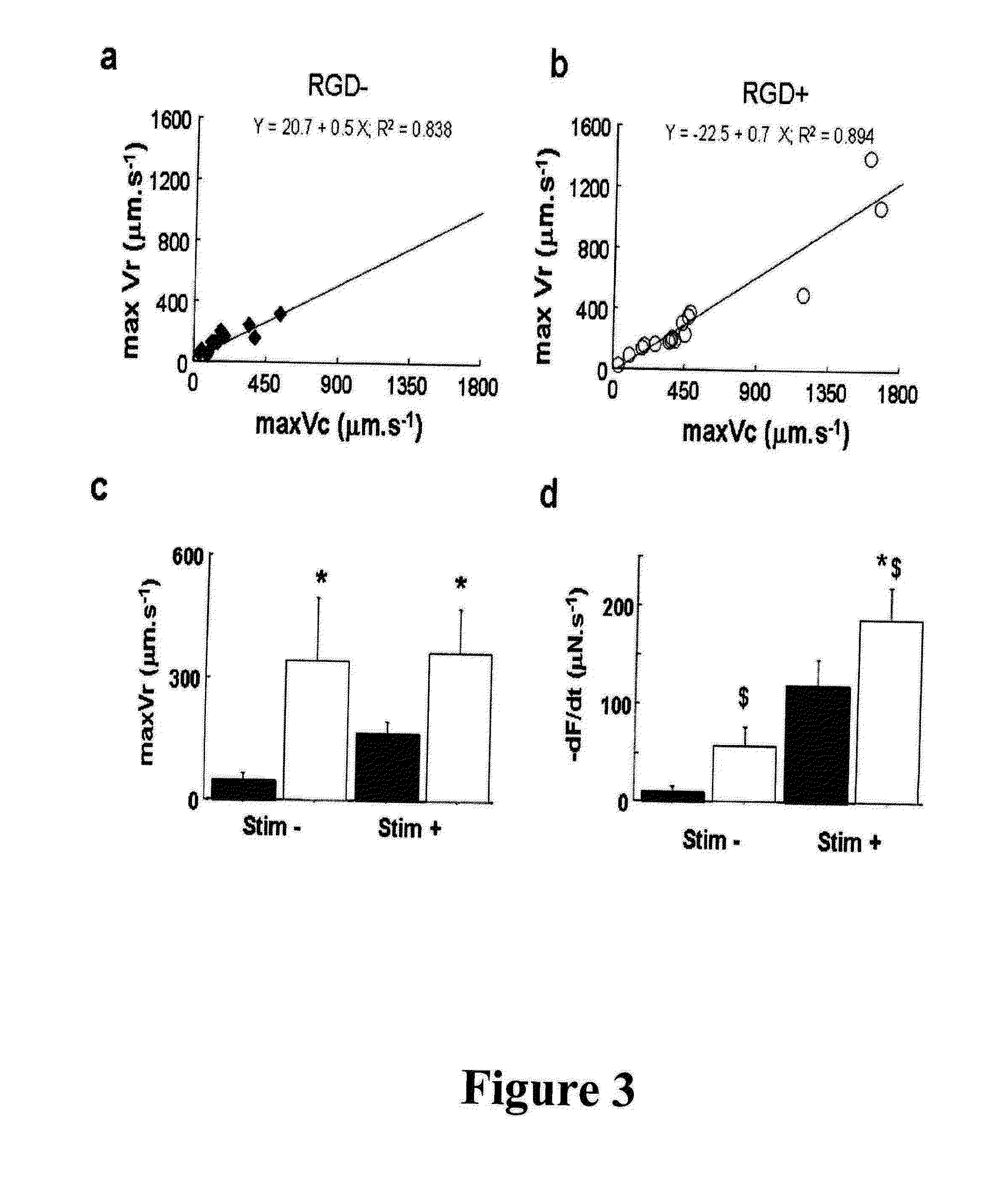
[0105]Angiogenesis induction in vitro by associating endothelial cells in a functionalized matrix with adhesion molecules:
[0106]In another type of preparation, cells of interest such as endothelial cells (mature or progenitor cells) may be transplanted into the functionalized 3D-collagen matrix with adhesion molecules, advantageously the RGD moiety, in the presence of or in the absence of contractile cells or other types of cells as for example fibroblasts, keratinocytes, contractile cells, genetically modified cells, etc. which may also be used independently. This support will promote the survival and the differentiation of the associated cell population as would do endothelial cells. This cell population may be used in association with contractile cells or independently. Such support may sometimes be also used without any initially associated cell population, the cells colonizing subsequently the support. However it has been demonstrated that associating endothelial cells does pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore size | aaaaa | aaaaa |
| Dielectric strength | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com