Coated implantable medical device method
a medical device and coating technology, applied in the field of human and veterinary medical devices, can solve the problems of stenosis or occlusion of blood vessels, blood vessel walls can be disturbed or injured, stenosis or occlusion,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
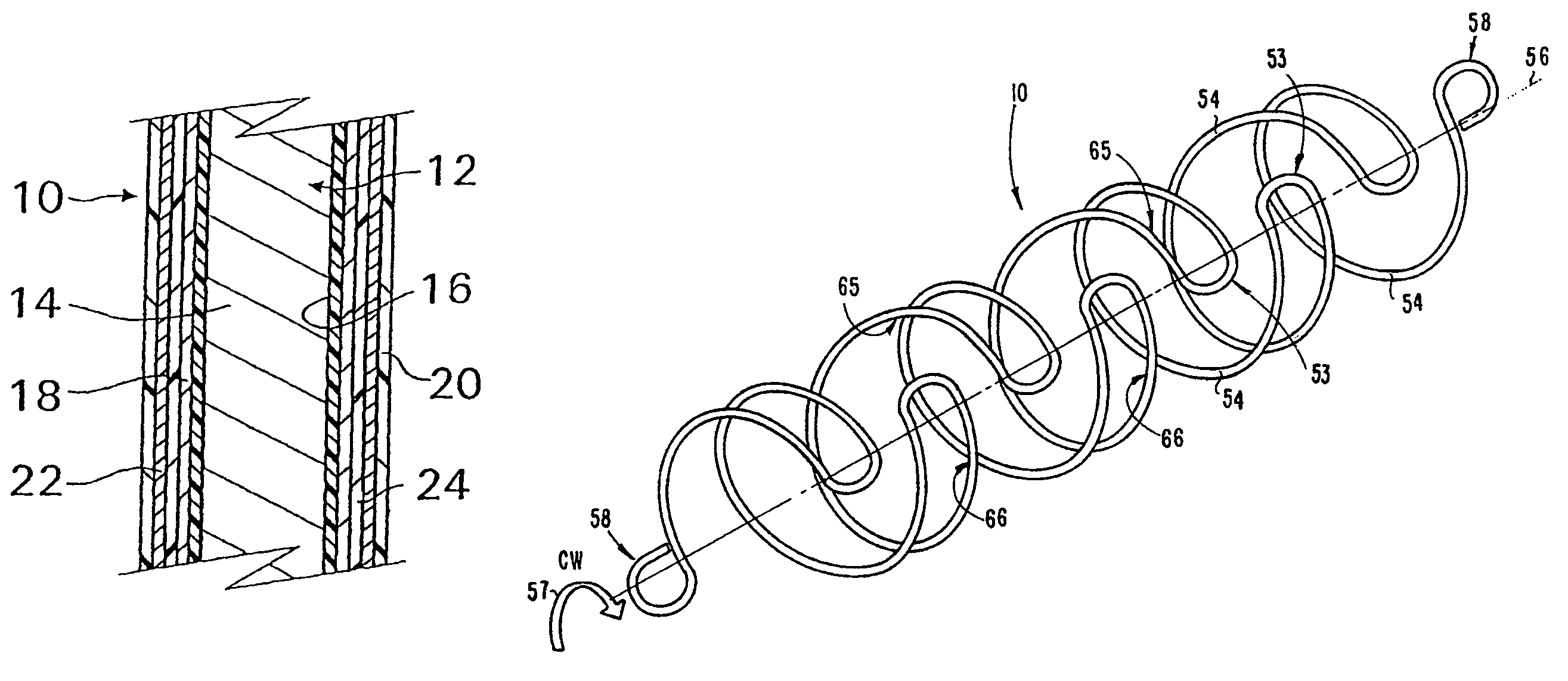
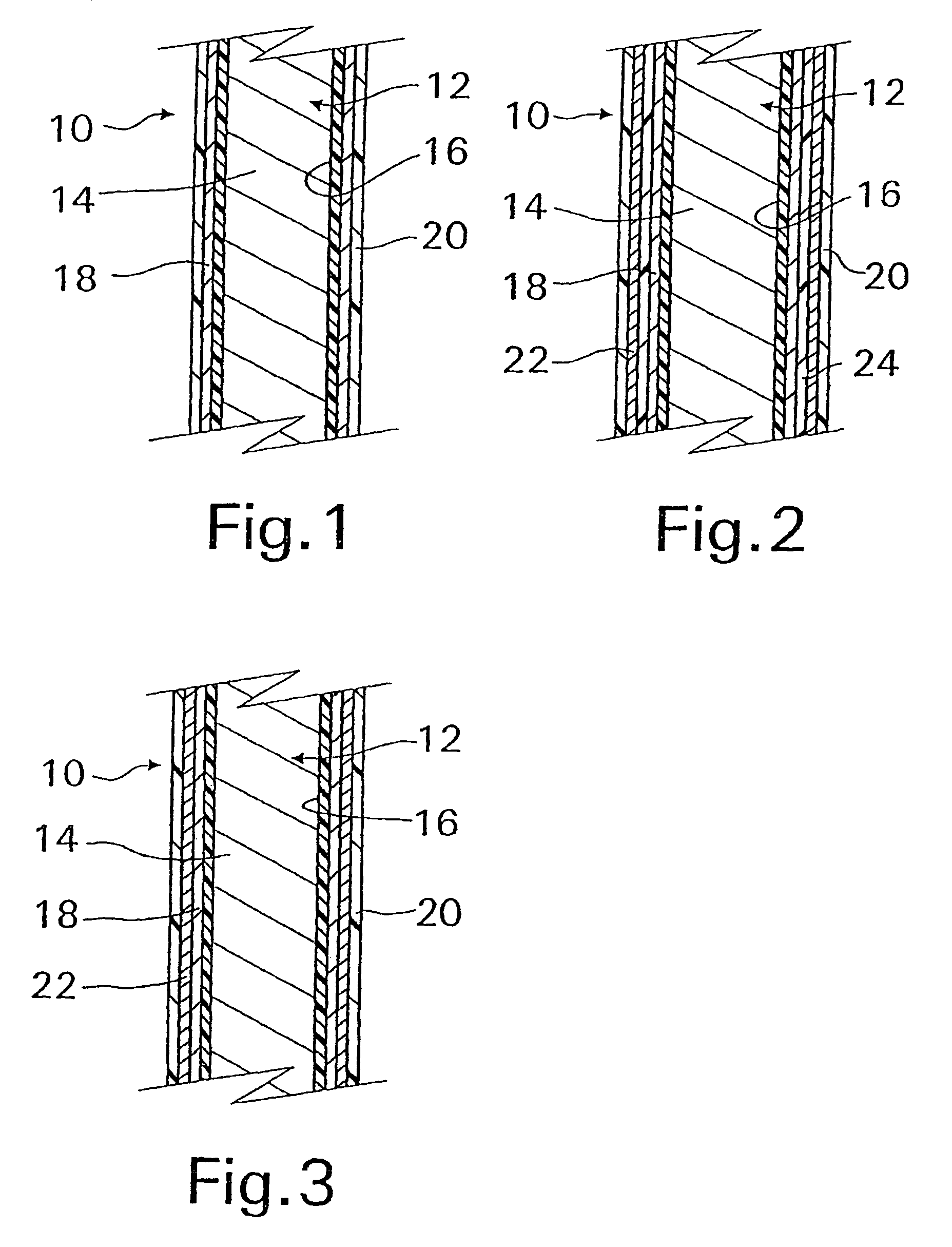
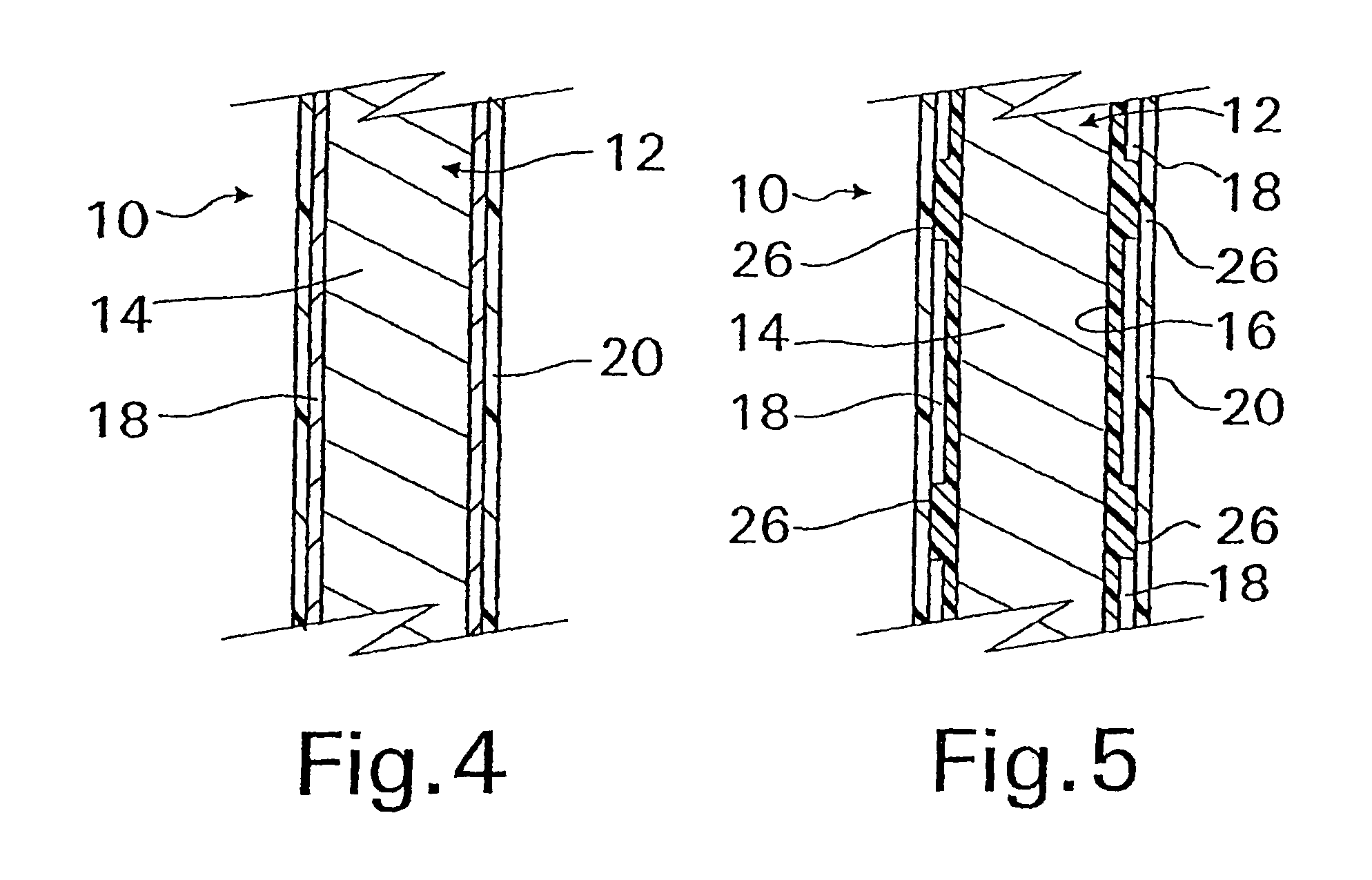
[0045]With reference now to FIG. 1, an implantable medical device 10 in accordance with the present invention is shown and first comprises a structure 12 adapted for introduction into a human or veterinary patient. “Adapted” means that the structure 12 is shaped and sized for such introduction. For clarity, only a portion of the structure 12 is shown in FIG. 1.
[0046]By way of example, the structure 12 is configured as a vascular stent particularly adapted for insertion into the vascular system of the patient. However, this stent structure can be used in other systems and sites such as the esophagus, trachea, colon, biliary ducts, urethra and ureters, subdural among others. Indeed, the structure 12 can alternatively be configured as any conventional vascular or other medical device, and can include any of a variety of conventional stents or other adjuncts, such as helical wound strands, perforated cylinders, or the like. Moreover, because the problems addressed by the present inventi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com