Methods for producing isomers of muconic acid and muconate salts
a technology of muconate salt and isomer, which is applied in the field of biological production of muconate to achieve the effects of reducing the number of isomers, improving the solubility of isomers, and improving the purity of isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
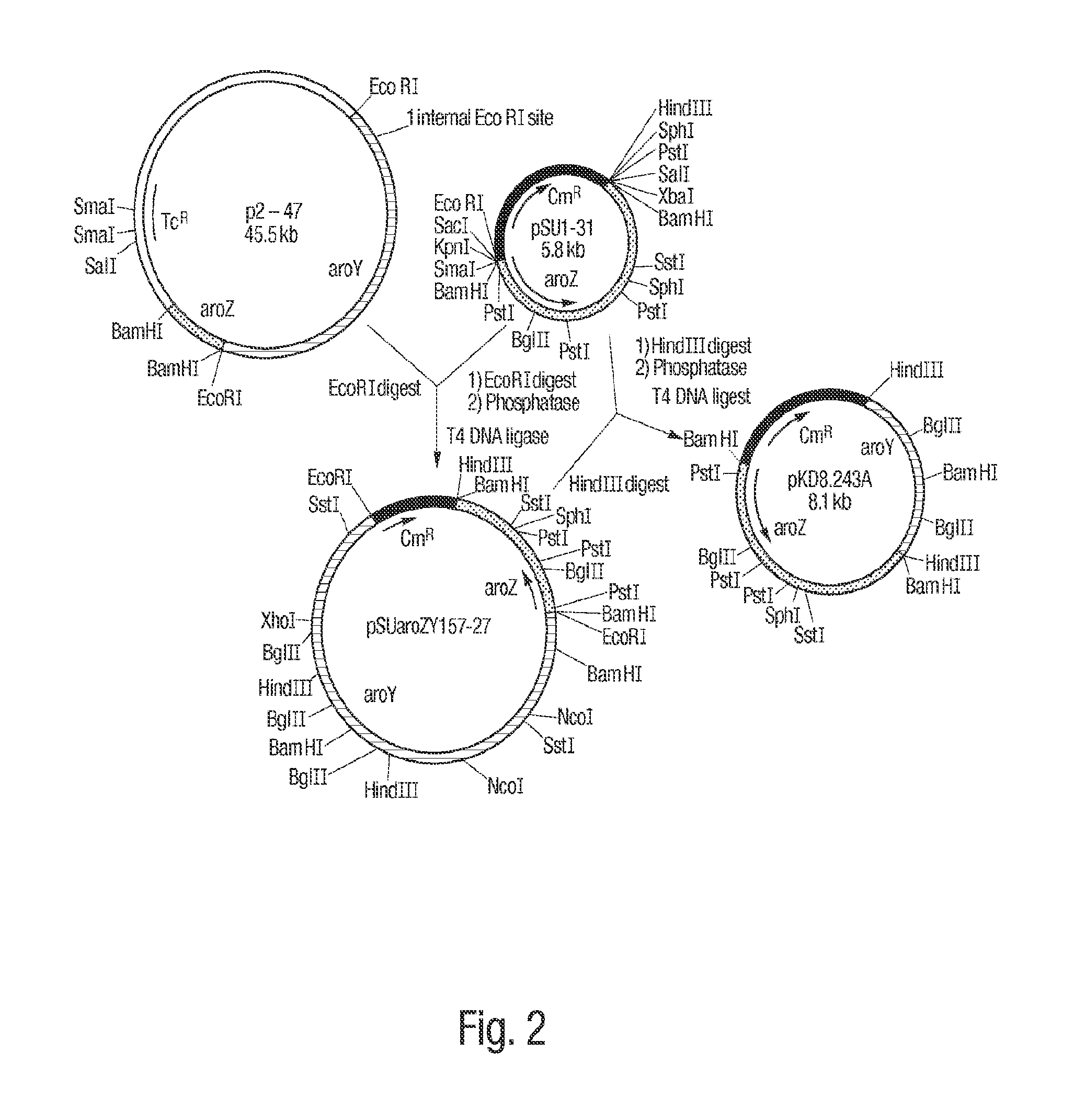
[0085]The gene encoding DHS dehydratase, designated aroZ, was isolated from a genomic library of Klebsiella pneumoniae DNA. Genomic DNA was purified from K. pneumoniae strain A170-40 and partially digested with BamH I to produce fragments in the range of 15 kb to 30 kb. The resulting DNA fragments were ligated to cosmid pLAFR3 which had previously been digested with BamH I and subsequently treated with calf intestinal alkaline phosphatase. pLAFR3 is a tetracycline resistant cosmid possessing the RK2 replicon. Ligated DNA was packaged using Packagene Packaging System (Promega), and the resulting phage particles were used to infect E. coli DH5α / pKD136. Plasmid pKD136 is a pBR325-based vector (pMB1 origin of replication) containing genes which encode transketolase (tkt), DAHP synthase (aroF), and DHQ synthase (aroB) as well as an ampicillin resistance gene. Colonies which were resistant to both tetracycline and ampicillin were subsequently plated onto chromogeni...
example 2
Confirmation of the Cloning of the aroZ Gene
[0086]Confirmation that cosmid p5-87 contained the aroZ gene relied on the fact that transformation of an E. coli strain which typically converts D-glucose into DHS could further convert DHS into protocatechuic acid. E. coli AB2834 accumulates DHS in the culture supernatant due to a mutation in the aroE gene, which encodes shikimate dehydrogenase. Conversion of D-glucose to DHS is maximized when AB2834 is transformed with pKD136. AB2834 was co-transformed with pKD136 and p5-87 to produce colonies that were resistant to both ampicillin and tetracycline. One liter of LB medium (4 L Erlenmeyer flask) was inoculated with an overnight culture (5 mL) of AB2834 / pKD136 / p5-87. The culture was grown at 37° C. for 8 h with agitation (250 rpm). The cells were then harvested and resuspended in one liter (4 L Erlenmeyer flask) of minimal M9 medium containing glucose (10 g L), shikimic acid (0.04 g L), ampicillin (0.05 g L), and tetracycline (0.013 g L)....
example 3
Subcloning of the aroZ Gene
[0087]In an effort to minimize the size of the aroZ-encoding insert plasmid p5-87 was digested with BamH I and the resulting fragments were ligated to vector pSU19 which had previously been digested with BamH I and treated with phosphatase. Plasmid pSU19 contains the p15A replicon and the gene which imparts resistance to chloramphenicol. Following transformation of the ligation products into E. coli DH5α / pKD136, the resulting ampicillin and chloramphenicol resistant colonies were screened as described in Example 1 for the ability to turn chromogenic minimal medium agarose plates containing p-toluidine and ferric citrate brown. Using this technique, plasmid pSU1-31 was isolated which consisted of a 3.5 kb BamH I insert contained in pSU19. When AB2834 / pKD136 / pSU1-31 was grown on a 1 L scale under conditions similar to those described in Example 1, 1H NMR analysis of the culture supernatant of indicated that 11 mM protocatechuic acid accumulated extracellular...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com