Method for producing potassium stannate
A production method and technology of potassium stannate, applied in chemical instruments and methods, tin compounds, sodium/potassium compounds, etc., can solve problems such as increased production cost, difficult removal of impurities, difficult operation, etc., and reduce anti-corrosion requirements and reduce production costs. The effect of reduction and obvious process advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
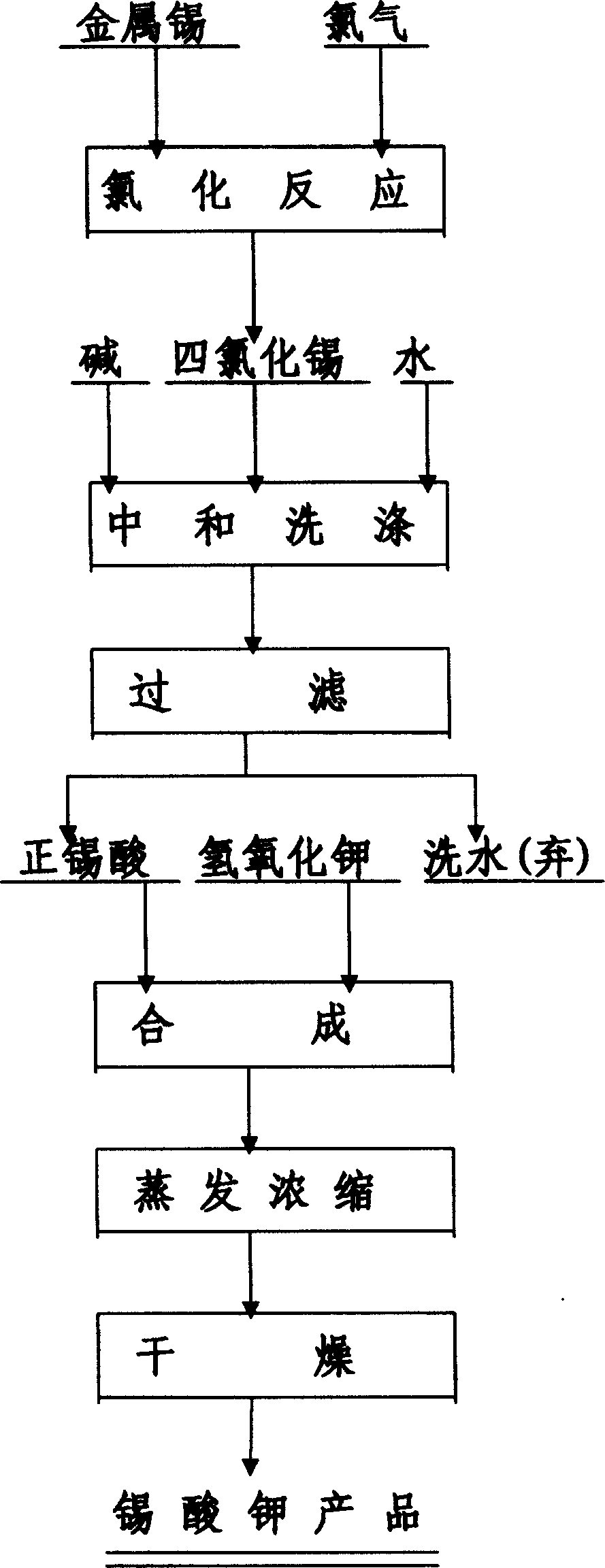
[0020] Under airtight conditions, chlorine gas is added to metal tin, and the chlorination reaction generates tin tetrachloride. Take 120Kg of ammonium bicarbonate, 240Kg of concentrated ammonia water, and 600Kg of water, mix, stir, slowly add 300Kg of anhydrous tin tetrachloride, adjust the amount of tin tetrachloride, control the neutralization end point pH value of 6-7, temperature 40℃~60 ℃. After the neutralization operation is completed, add water to wash the sediment 3 times, add 3000Kg of water each time, stir for 10 minutes, clarify for 8 hours, and then siphon the supernatant to discharge. The washed precipitate is filtered by a centrifuge to obtain orthostannic acid. Weigh, take samples to test the tin content, calculate the weight of tin contained in the stannic acid, weigh the potassium hydroxide according to the molar ratio of potassium hydroxide to tin 2:1, add to the stannic acid, stir and mix. Heating, evaporating and concentrating, adding the solid to a dryer with...
example 2
[0022] Slowly add 600Kg of anhydrous tin tetrachloride to 1200Kg of water, and then add about 750Kg of ammonium bicarbonate, keep stirring and heating during the neutralization process, and keep the reaction temperature between 20°C and 40°C, adjust the amount of ammonium bicarbonate added, and control the neutralization end pH The value is 3.5 to 5. After the neutralization operation is completed, add water to wash the precipitate 3 times, each time add 6000Kg of water, stir for 10 minutes, clarify for 8 hours, and then siphon the supernatant to discharge. After the precipitate is filtered by centrifugation, stannic acid is obtained. Weigh, sample and test the tin content, calculate the weight of tin contained in the stannic acid, weigh the potassium hydroxide into the stannic acid according to the molar ratio of potassium hydroxide to tin 2:1, stir and mix. Heating, evaporating and concentrating, adding the solid to a dryer with stirring, after drying, the finished product of po...
example 3
[0024] Slowly add 600Kg of anhydrous tin tetrachloride to 600Kg of water, cool to room temperature, stir, and add about 600Kg of concentrated ammonia water to it. During the neutralization process, keep stirring and cooling, keep the reaction temperature at 60℃~80℃, and adjust the addition of concentrated ammonia water. Control the neutralization end point pH value of 7-8. After the neutralization operation is completed, add water to wash the precipitate 3 times, each time add 6000Kg of water, stir for 10 minutes, clarify for 8 hours, and then siphon the supernatant to discharge. After the precipitate is filtered by centrifugation, stannic acid is obtained. Weigh, sample and test the tin content, calculate the weight of tin contained in the stannic acid, weigh the potassium hydroxide into the stannic acid according to the molar ratio of potassium hydroxide to tin 2:1, stir and mix. Heating, evaporating and concentrating, adding the solid to a stirring dryer, after drying, 685Kg of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com