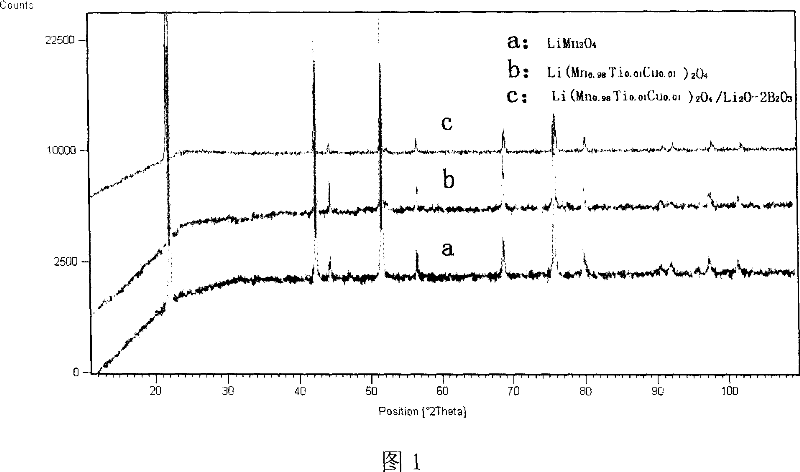
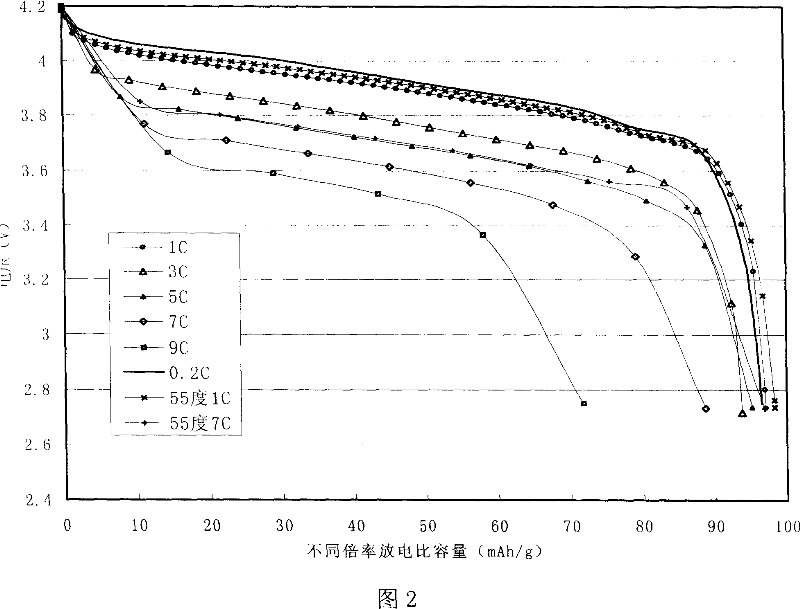
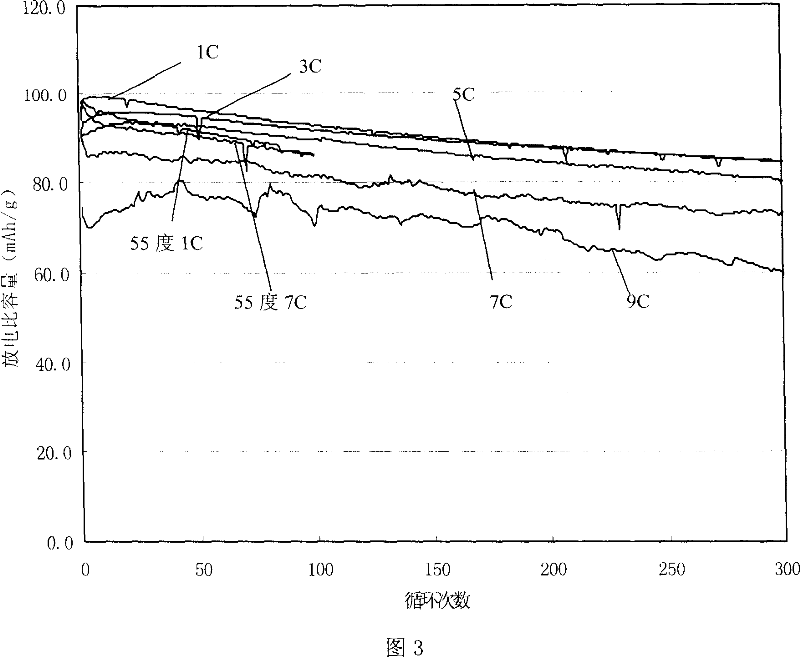
Method for preparing modified spinelle manganic acid lithium material and lithium secondary battery
A spinel lithium manganate, lithium secondary battery technology, applied in the field of electrochemistry, can solve problems such as capacity decay, and achieve the effects of improving compatibility, easy industrialization, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh LiOH·H 2 O 1Kg, and according to the mass ratio of 1.5:1 (material weight / ball weight), take zirconia balls and add them to the ball mill cylinder for ball milling for 2h, then dry them for later use, and weigh LiOH·H 2 O (ball milled), electrolytic manganese dioxide (EMD), titanium dioxide (TiO 2 ) and copper oxide (CuO) were put into a ball mill cylinder, and zirconia balls of about 1 times the weight were added to the ball mill for 4 hours, then calcined at 500°C for 5h, and then continued to be calcined at 800°C for 10h, cooled naturally to room temperature, taken out and ground, and sieved ( 300 mesh or so) to obtain preliminary material A1. Moore Billy 2 O-2B 2 o 3 / Preliminary material = 0.5% Weigh LiOH·H 2 O and H 3 BO 3 Put it in a beaker, and add about 20 times the mass of methanol to dissolve it to obtain LiOH·H 2 O-H 3 BO 3 / methanol coating solution B1. The amount of methanol added is generally based on the principle of economy and ensures...
Embodiment 2
[0039] Weigh Li 2 CO 3 1Kg, and according to the mass ratio of 1.5:1 (material weight / ball weight), take zirconia balls and add them to the ball mill cylinder for ball milling for 2 hours, then dry them for later use, and weigh Li 2 CO 3 (ball milled), electrolytic manganese dioxide (EMD), chromium oxide (Cr 2 o 3 ) and aluminum nitrate (Al(NO 3 ) 3 ) into a ball mill cylinder, and added about 1 times the weight of zirconia balls for 4 hours, then calcined at 450°C for 8 hours, then continued to sinter at 850°C for 8 hours, cooled naturally to room temperature, took out and ground, sieved (about 300 mesh) to obtain a preliminary Material A1. Weigh an appropriate amount of polyvinyl alcohol PVA by molar ratio C / preliminary material = 0.6%, put it in a beaker and add about 20 times of ethanol to dissolve to obtain PVA coating solution B1. The amount of ethanol added is also based on the principle of economy and ensures the Fully dispersed to determine. Under the conditi...
Embodiment 3
[0042] Weigh anhydrous LiNO 3 1Kg, and according to the mass ratio of 1.5:1 (material weight / ball weight), take zirconia balls and add them to the ball mill cylinder for ball milling for 2 hours, then dry them for later use, and weigh LiNO respectively according to the molar ratio of 1.05:1.94:0.01:0.04 3 (ball milled), electrolytic manganese dioxide (EMD), gallium oxide (Ga 2 o 3 ) and magnesium hydroxide (Mg(OH) 2 ) into a ball mill cylinder, and add about 1 times the weight of zirconia balls for ball milling for 4 hours, then calcined at 600°C for 4 hours, then continued to calcine at 800°C for 14 hours, cooled naturally to room temperature, took out and ground, sieved (about 300 mesh) to obtain a preliminary Material A1. Lithium carbonate by molar ratio: tricobalt tetroxide: preliminary material = 3: 2: 100 Weigh lithium carbonate, tricobalt tetroxide and place in a beaker, and add about 20 times of mass multiple of acetone to dissolve to obtain lithium carbonate, tric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com