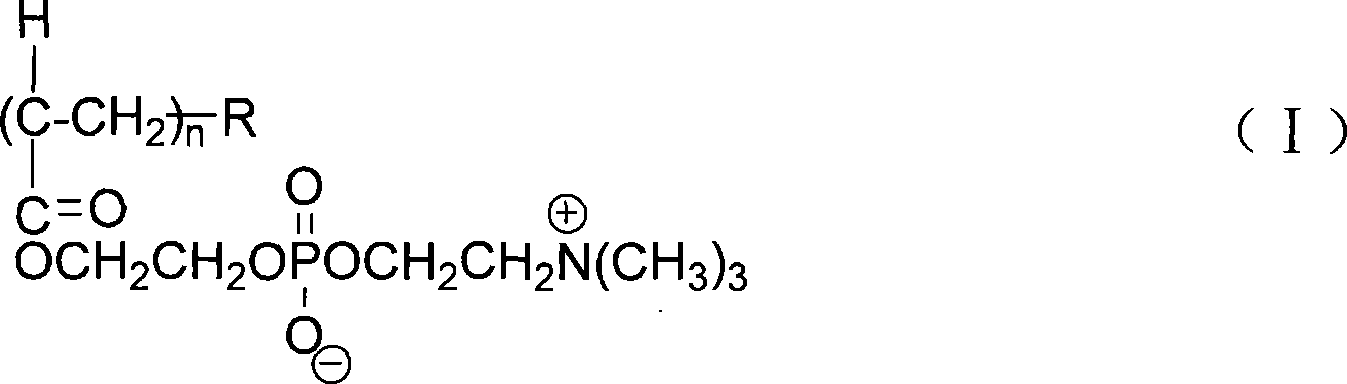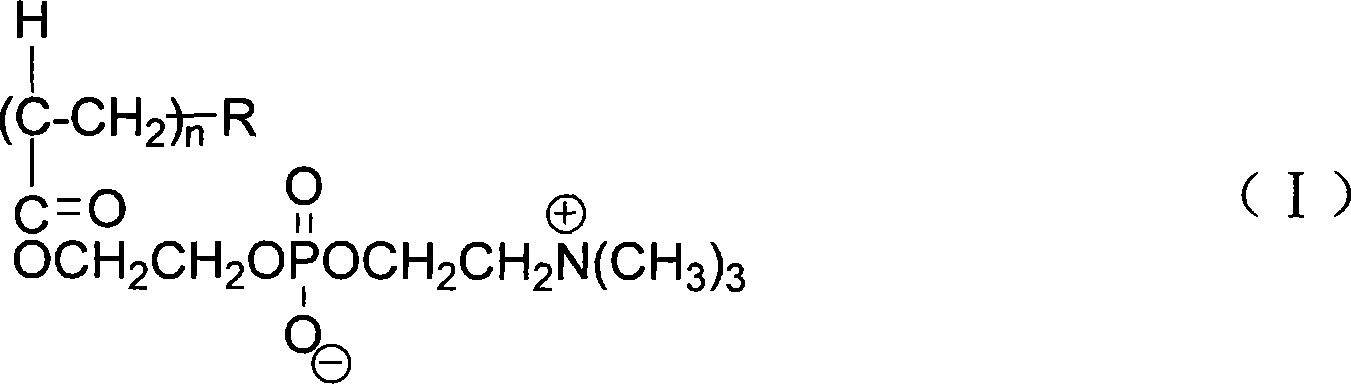Acid choline biomimetic polymers coated carbon-nano tube and preparation method thereof
A carbon nanotube and phosphorylcholine-based technology, applied in the interdisciplinary field of disciplines, can solve the problems of stability, dispersion and biocompatibility, the destruction of the physical structure and mechanical properties of carbon nanotubes, and the limitation of carbon nanotubes. Regardless of practical application and other issues, to achieve good solubility, stable non-covalent interaction, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) In a 100mL three-neck flask, add 50mL of dichloromethane, then add 1g of cholesterol, and in the case of argon flow at 10°C, add 4.6g of bromoisobutylyl bromide dropwise to the solution, and react for 5hr to obtain cholesterified Atom transfer radical initiator.
[0031] 2) In a polymerization tube, add 10 mL of methanol, 10 mg of the above atom transfer radical polymerization initiator, and 1 g of phosphorylcholine methacrylate. After the polymerization tube was deoxygenated, 10 mg of cuprous chloride and 10 mg of bipyridine were added to it, the solution was dark brown, and reacted at 25° C. for 8 hours to obtain a phosphorylcholine amphiphilic polymer containing hydrophobic terminal cholesterol.
[0032] 3) In the Erlenmeyer flask, add 1g of carbon nanotubes and 10g of an aqueous solution of phosphorylcholine amphiphilic polymer (I) containing 1wt% cholesterol-containing degree of polymerization n=50, and then use a 40kHz ultrasonic wave to the mixed solution Dis...
Embodiment 2
[0038] 1) In a 100mL three-necked flask, add 50mL of dichloromethane, then add 1g of pyrene, and in the case of argon flow at 25°C, add 10g of bromoisobutylyl bromide dropwise to the solution, and react for 0.1hr to obtain pyrene-containing Atom transfer radical initiator.
[0039] 2) In a polymerization tube, add 10 mL of methanol, 10 mg of the above atom transfer radical polymerization initiator, and 50 mg of phosphorylcholine methacrylate. After the polymerization tube was deoxygenated, 20 mg of cuprous chloride and 50 mg of bipyridine were added to it, and the solution was dark brown. After reacting at 10° C. for 10 hr, a phosphorylcholine amphiphilic polymer containing pyrene at the hydrophobic end was obtained.
[0040] 3) In the Erlenmeyer flask, add 50g of carbon nanotubes and 2000g of an aqueous solution containing 0.05wt% of pyrene-containing phosphorylcholine amphiphilic polymer (I) with a degree of polymerization n=50, and then use a 40 kHz Ultrasonic ultrasonic d...
Embodiment 3
[0046]1) In a 100mL three-necked flask, add 50mL of dichloromethane, then add 1g of PLA, and add 0.1g of bromoisobutylyl bromide dropwise to the solution under the condition of argon flow at 50°C, and react for 10 hours to obtain PLA-containing atoms Transfer radical initiator.
[0047] 2) In a polymerization tube, add 10 mL of methanol, 10 mg of the above atom transfer radical polymerization initiator, and 0.5 g of phosphorylcholine methacrylate. After the polymerization tube was deoxygenated, 50 mg of cuprous chloride and 40 mg of bipyridine were added to it, the solution was dark brown, and reacted at 50° C. for 0.1 hr to obtain a phosphorylcholine amphiphilic polymer containing hydrophobic terminal PLA.
[0048] 3) In the Erlenmeyer flask, add 50g of carbon nanotubes and 2000g of an aqueous solution containing 0.05wt% PLA-containing phosphorylcholine amphiphilic polymer (I) with a degree of polymerization n=50, and then use a 40 kHz Ultrasonic ultrasonic dispersion for 4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aspect ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com