Silicon-acrylic tri-block copolymer with low surface energy and preparing method thereof
A silicon-acrylic triblock and block copolymer technology, which is applied in the field of silicon-acrylic tri-block copolymers with low surface energy and its preparation, can solve the problem of many side reactions of anionic polymerization, difficulty in separation and purification, and inability to contain oxygen And water and other problems, to achieve the effect of narrow molecular weight distribution, less side reactions, and low surface energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
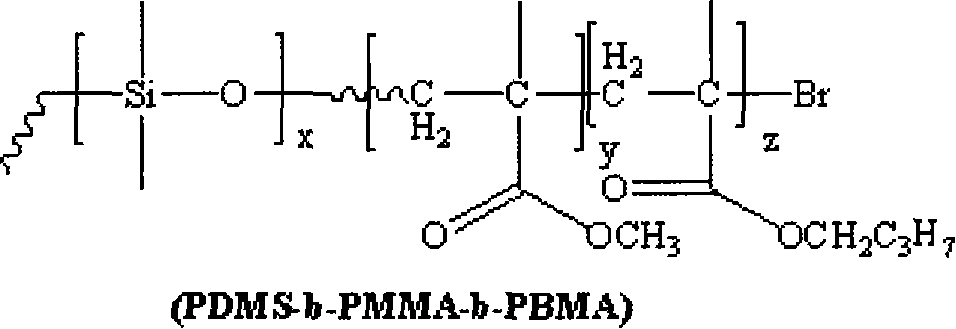
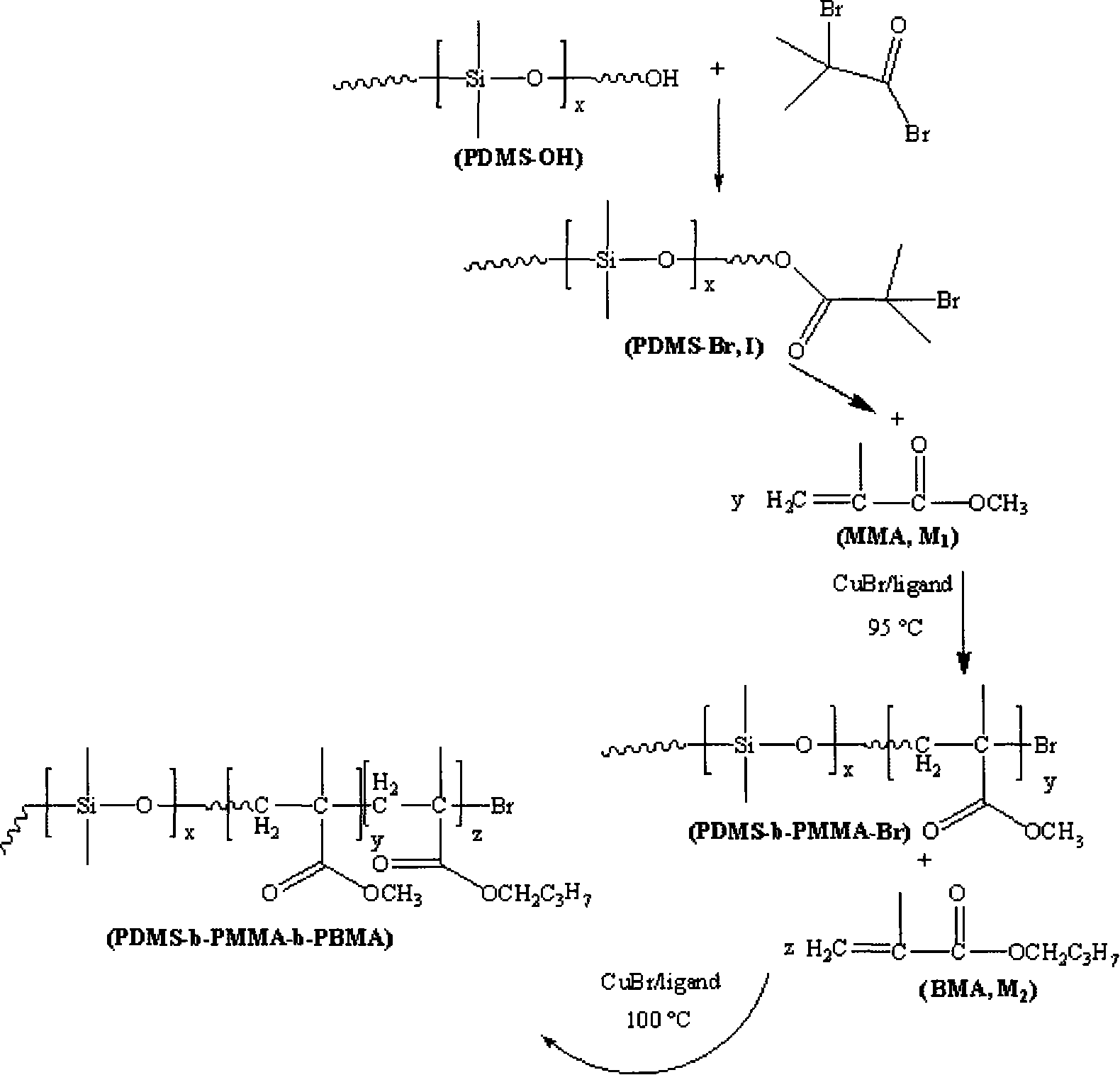
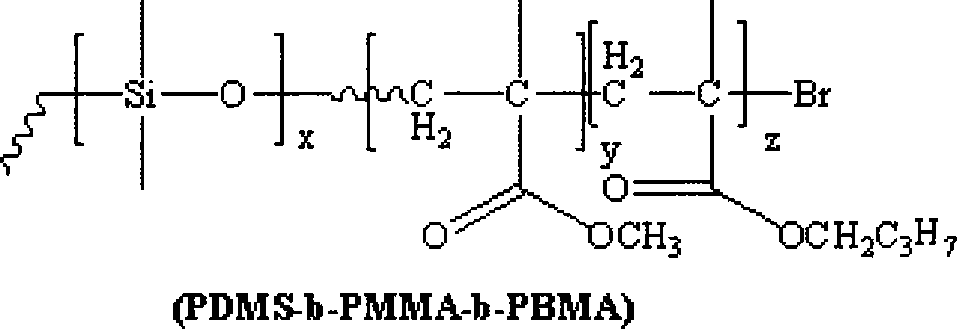
[0023] 1) Under the protection of a nitrogen atmosphere, 1 part of monomethanol-terminated polydimethylsiloxane, 1.5 parts of 2-bromoisobutyryl bromide and 2.5 parts of triethylamine are reacted at 5°C. Then, the above solution was stirred and reacted for 30 hours. After the reaction, it was filtered, the filtrate was distilled under reduced pressure to remove the solvent, and the filtrate was dissolved in dichloromethane, washed with saturated sodium bicarbonate solution several times, separated, and the organic layer Dry with anhydrous magnesium sulfate to remove water, then filter, and finally distill the filtrate under reduced pressure to remove the dichloromethane solvent to obtain an oily yellow macroinitiator;
[0024] 2) The reaction system must be strictly deoxygenated, using 1 part of macromolecule as initiator, 1 part of cuprous bromide as catalyst, and 2 parts of N-(n-propyl)-2-pyridine methylamine as catalyst ligand React with 10 parts of methyl methacrylate as the mo...
Embodiment 2
[0027]1) Under the protection of argon atmosphere, 1 part of monomethanol-terminated polydimethylsiloxane, 4.0 parts of 2-bromoisobutyryl bromide and 5.5 parts of triethylamine are reacted at 10°C. Then, the above solution was stirred and reacted for 20 hours. After the reaction, it was filtered, the filtrate was distilled under reduced pressure to remove the solvent, and then dissolved in dichloromethane, washed with saturated sodium bicarbonate solution several times, separated, and the organic layer Dry with anhydrous magnesium sulfate to remove water, then filter, and finally distill the filtrate under reduced pressure to remove the dichloromethane solvent to obtain an oily yellow macroinitiator;
[0028] 2) Except that the ligand is 1,1,4,7,7-pentamethyldivinyltriamine, the monomer is 20 parts, and the reaction temperature is 50°C, the others are the same as step 2) in Example 1;
[0029] 3) Except that the reaction temperature is 60°C, the others are the same as step 3) in E...
Embodiment 3
[0031] 1) Except that the reaction temperature is 15h, everything else is the same as step 1) in Example 1;
[0032] 2) Except that the monomer is 30 parts, the others are the same as step 2) in Example 2;
[0033] 3) Except that the reaction temperature is 70°C and the ligand is 1,1,4,7,7-pentamethyldivinyltriamine, the others are the same as step 3) in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com