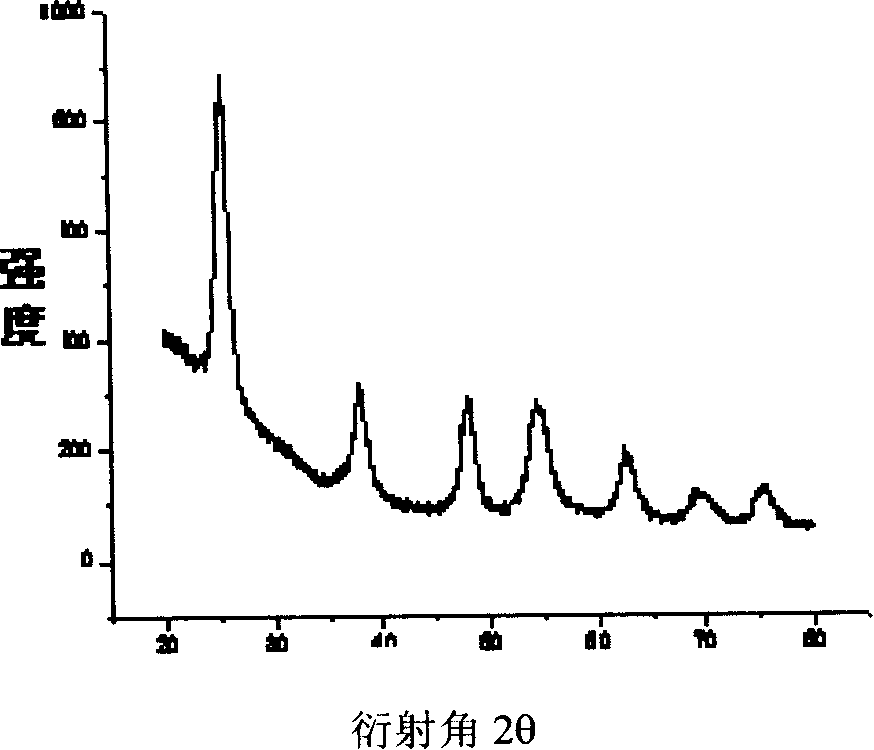
Semiconductor TiO2 photocatalyst of surface modified cocatalyst, preparing method and uses thereof
A surface modification and photocatalyst technology, which is applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, carbon-based compound preparation, etc., can solve the problem of poor selectivity, pore mass transfer limitation, inability to obtain aldehydes or The problem of ketone target products and other problems is achieved, and the effect of mild preparation and reaction conditions and long life is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
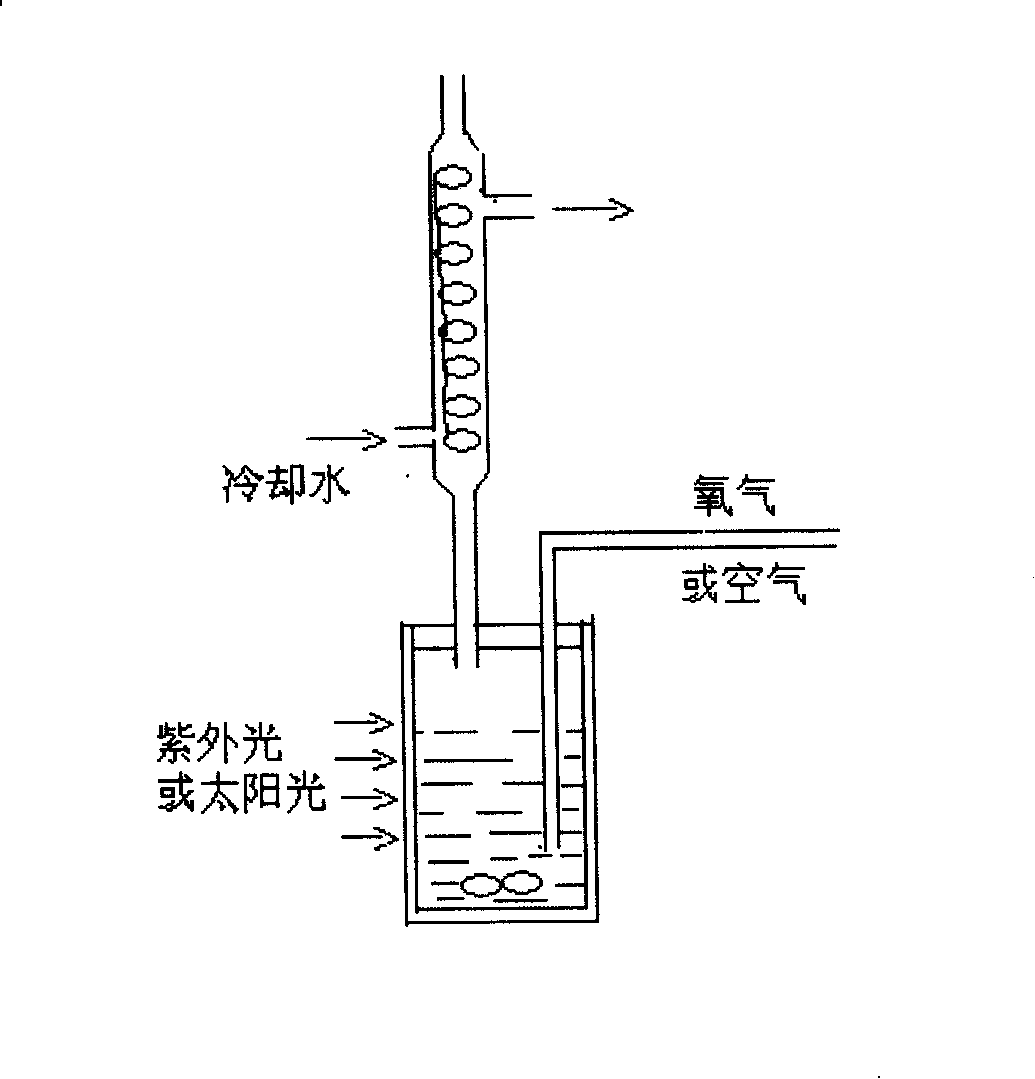
[0040] 0.01g of anatase TiO 2 Suspend in 3ml trifluorotoluene solvent, add 5×10 -5 Mole of co-catalyst (2,2,6,6-tetramethylpiperidine nitroxide radical, TEMPO for short, structure of co-catalyst structure 1), stirred at room temperature (25℃) for 1h, then separated by filtration and dried in vacuum Solid catalyst, the prepared catalyst is a uniform white granular substance. Take 0.003g of the above catalyst in the photoreactor (see attached figure 2 ), add 1.5ml of trifluorotoluene solvent and substrate benzyl alcohol and 0.1ml of various primary or secondary alcohols (see Table 1 and Table 2) to make TiO 2 The percentage of the total moles of the photocatalyst / cocatalyst and the moles of the substrate alcohol is between 1-25% (the same in the following examples). After stirring evenly, pass in the dried air, turn on the xenon lamp to irradiate the reaction, 1000W, The reaction temperature is 25°C, and the conversion rate of the substrate is monitored. When the conversion of the s...
Embodiment 2
[0058] The preparation of the catalyst was the same as that of Example 1, and according to the molar ratio of 1% (ie TiO 2 The percentage of the ratio of the photocatalyst / cocatalyst mole sum to the reactant alcohol mole ratio, the following examples are the same) TiO 2 Photocatalyst / Co-catalyst (2,2,6,6-Tetramethyl-4-one-1-oxo-piperidine radical, structure as co-catalyst structure 2, co-catalyst / TiO 2 The photocatalyst ratio is 0.05 mol promoter / g TiO 2 ) In the photoreactor, add dichloroethane solvent and reactant benzyl alcohol, stir evenly, pass in the dried air, turn on the xenon lamp to irradiate the reaction, monitor the conversion rate of the substrate, when the substrate conversion reaches more than 90% , Turn off the light source to end the reaction. The reaction product was measured with a gas-mass spectrometer.
Embodiment 3
[0060] The preparation of the catalyst is the same as in Example 1, and TiO is taken at a molar ratio of 25% 2 Photocatalyst / Co-catalyst (2,2,6,6-Tetraethyl-4-carboxy-1-oxo-piperidine radical, structure 3 of co-catalyst, co-catalyst / TiO 2 The photocatalyst ratio is 2.5×10 -3 Mole promoter / g TiO 2 ) In the photoreactor, add toluene solvent and n-hexanol, stir evenly, pass in dried pure oxygen, and irradiate the reaction under sunlight to monitor the conversion rate of the substrate. When the substrate conversion reaches more than 80%, Turn off the light source to end the reaction. The reaction product was measured with a gas-mass spectrometer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com