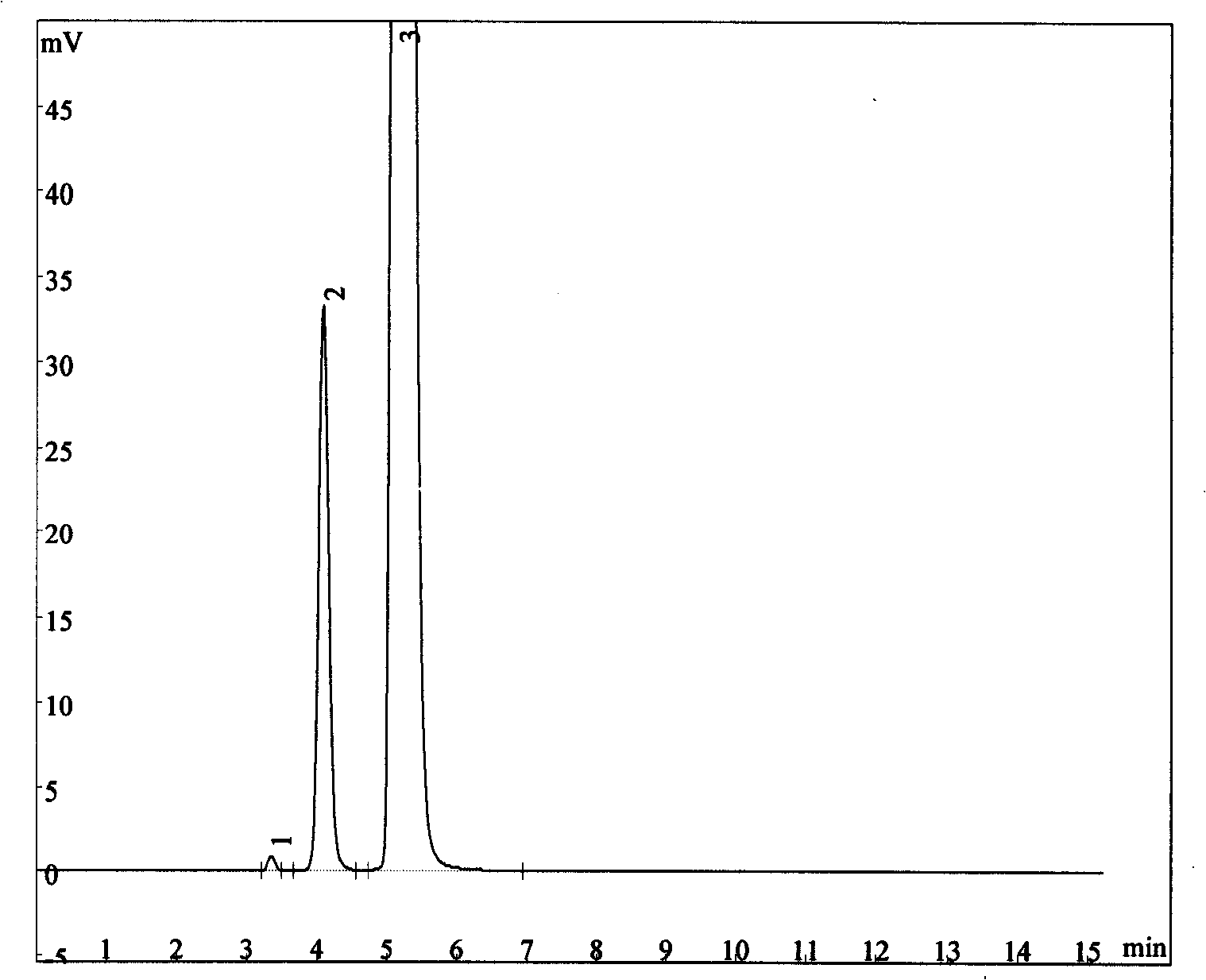
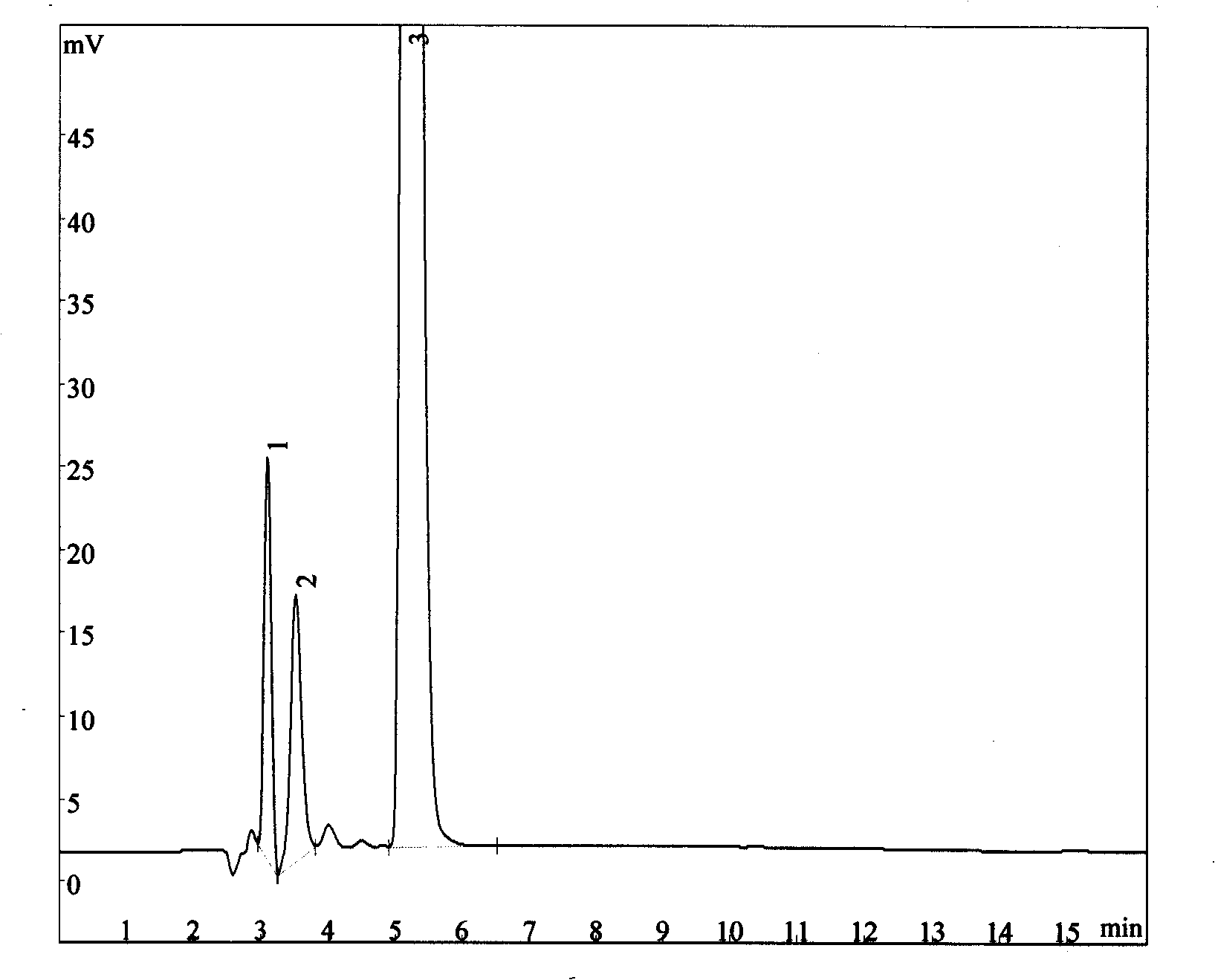
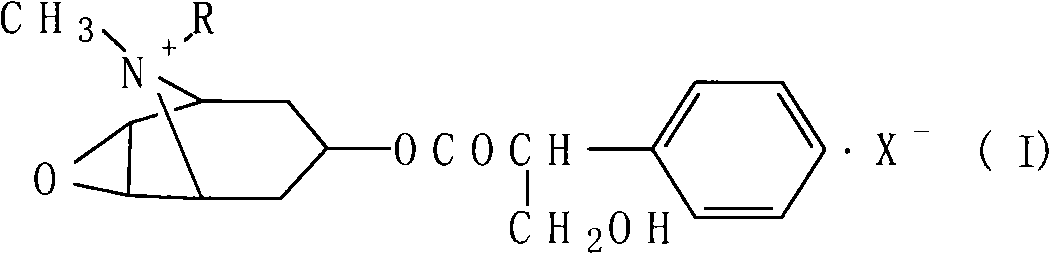Process for synthesizing and detecting scopolamine derivates
A technology of scopolamine and a synthesis method, which is applied in directions such as drug combinations, measuring devices, instruments, etc., can solve the problems of difficulty in separating by-products, affecting the accuracy of results, increasing by-products, etc., and achieving reduced energy consumption, high accuracy, and shortened reactions. effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the synthesis of citropium bromide
[0031] Add 33.9g scopolamine, 20.2g bromomethylcyclopropane, 21.2g sodium carbonate and 340ml acetonitrile in the dry reaction flask, under stirring condition, reflux for 4 hours, remove sodium carbonate with filter paper, and continue to stir and reflux the filtrate for 6 hours. After cooling, crystals were precipitated, filtered, and recrystallized with acetonitrile. 36.8 g of citropium bromide crystals were obtained, and the yield was 83.9%.
Embodiment 2
[0032] Embodiment 2: the synthesis of citropium bromide
[0033] Add 33.9g scopolamine, 20.2g bromomethylcyclopropane, 27.6g potassium carbonate and 340ml acetonitrile into the dry reaction flask, reflux for 4 hours under stirring, remove potassium carbonate with filter paper, and continue to stir and reflux the filtrate for 6 hours. After cooling, crystals were precipitated, filtered, and recrystallized with acetonitrile. 35.4 g of citropium bromide crystals were obtained, and the yield was 80.7%.
Embodiment 3
[0034] The synthesis of embodiment 3 Scopolamine butylbromide
[0035] Add 8.6g scopolamine, 3.6ml n-bromobutane, 4.2g sodium bicarbonate and 86ml acetonitrile in the dry reaction bottle, under stirring condition, reflux for 4 hours, remove sodium carbonate with filter paper, and continue to stir and reflux the filtrate for 6 hours. After cooling, crystals were precipitated, filtered, and recrystallized with acetonitrile. 8.5 g of scopolamine butylbromide crystals were obtained, with a yield of 77.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com