9-pyrene based fluorene structured linear conjugated polymer derivant material and preparation method thereof
A conjugated polymer, polymer technology, used in luminescent materials, chemical instruments and methods, semiconductor/solid-state device fabrication, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1: the synthesis of polymer I1 (wherein R 1 = R 2 , is n-octyl; R 3 to a hydrogen atom).
[0094] (1) 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxoborolan-2-yl)-9-phenyl-9-pyrenylfluorene (boron acid esters 1).
[0095] 2,7-Dibromo-9-phenyl-9-pyrenylfluorene (1.3 g, 2.2 mmol) was dissolved in anhydrous and oxygen-free THF (20 mL) under anhydrous and oxygen-free conditions, and cooled to -78°C. n-Butyllithium (1.6M solution in n-hexane) (5 mL, 8 mmol) was injected dropwise into the system with a syringe, and the system was stirred at -78°C for 1.5 h. Methyl triborate (2.5mL, 2.28g, 22mmol) was quickly injected into the system with a syringe at one time. After the system was stirred at -78°C for 1.5h, the temperature was slowly raised to room temperature, and the reaction was continued for 24h. Add 2M hydrochloric acid (50 mL) to acidify for 5 h, separate the layers, extract the aqueous phase with dichloromethane or ether, dry the combined organic phases with an...
Embodiment 2
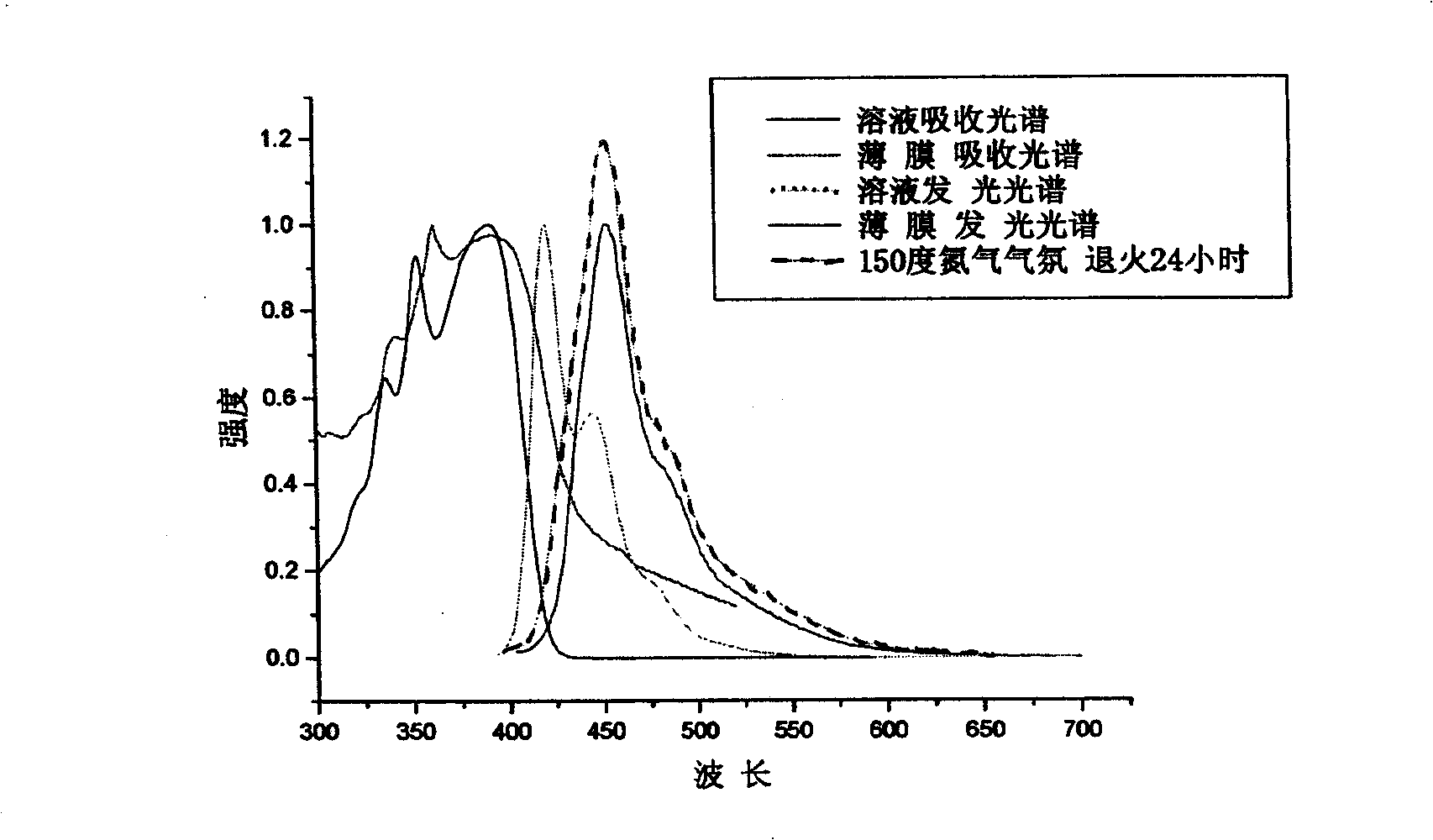
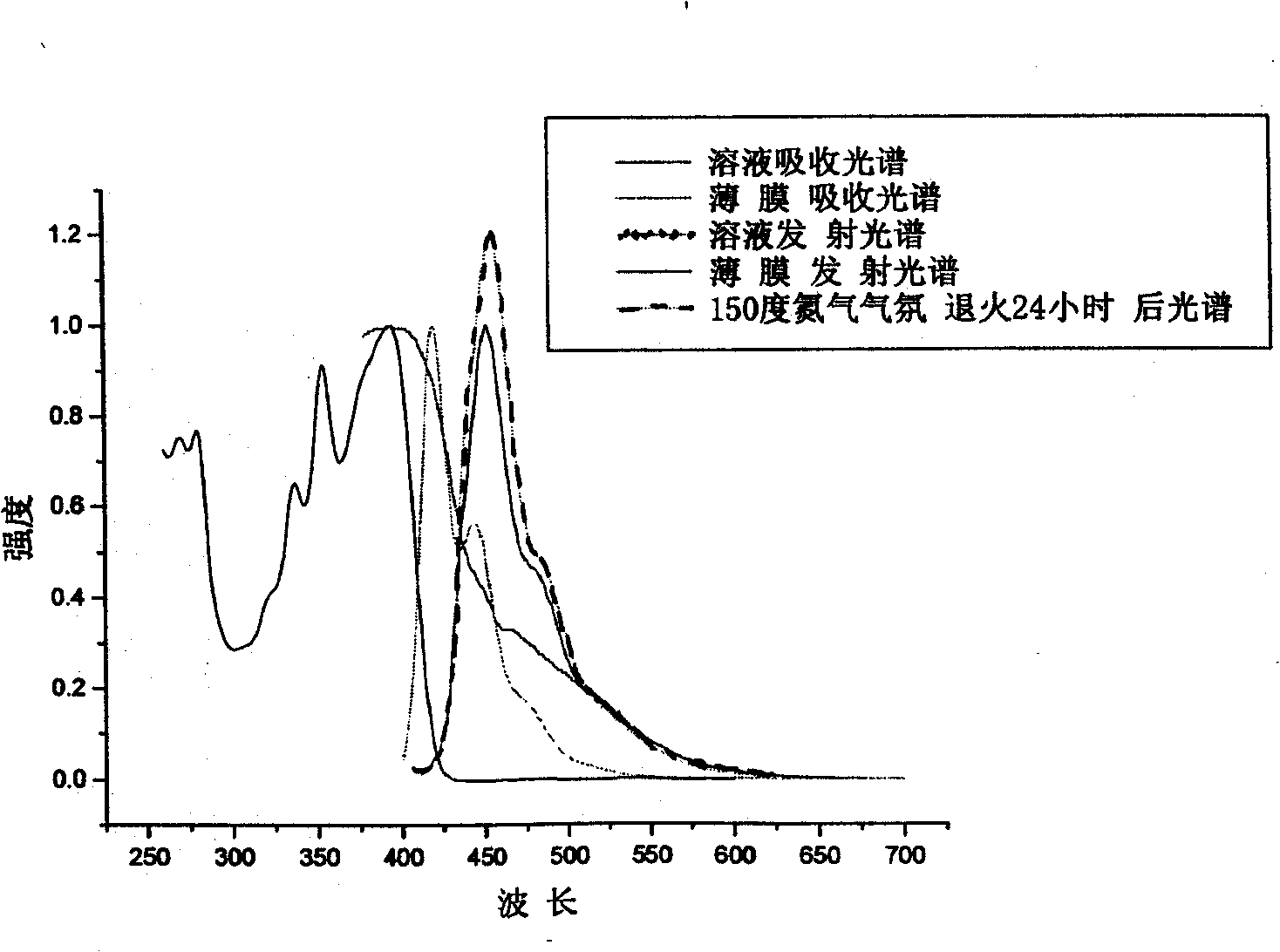
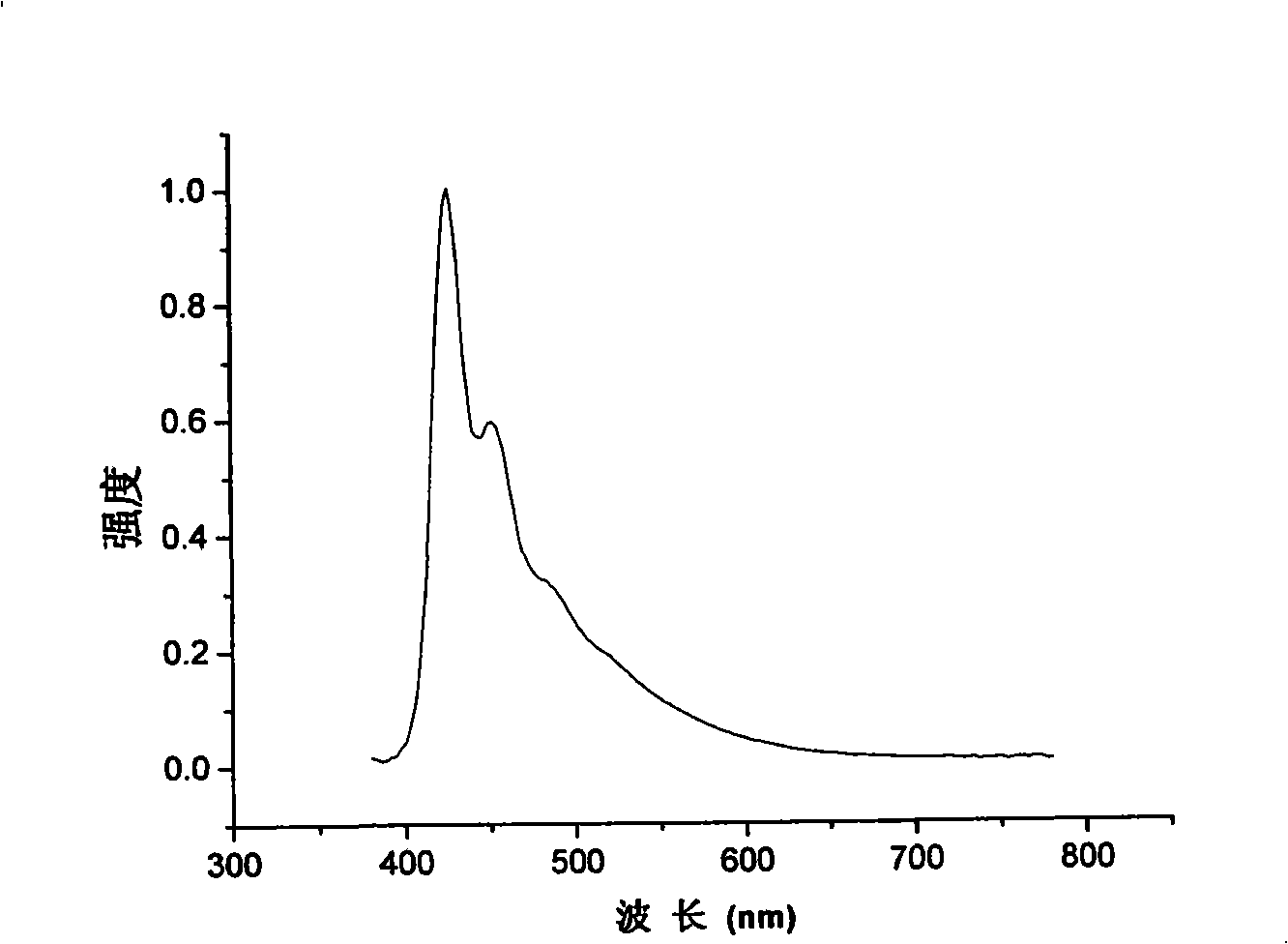
[0103] Example 2: Absorption spectra of solutions and thin films of polymer I1 (the product in Example 1), determination of photoluminescence spectroscopy.
[0104] Polymer I1 was dissolved in tetrahydrofuran solution, and the absorption and emission spectra were measured by Shimadzu UV-3150 ultraviolet-visible spectrometer and RF-530XPC fluorescence spectrometer. The photoluminescence spectrum is excited by the maximum absorption wavelength (391 nm) of ultraviolet absorption. The solid film is formed by dropping the solution on a transparent glass plate after the solvent evaporates. The fluorescence quantum efficiency of the solution is measured by 10 in cyclohexane -6 The 9,10-diphenylanthracene solution of M (quantum efficiency 0.9) was used as a standard to measure, and the measured value was 93%.
[0105] The polymer I1 solution has a maximum absorption peak of 391nm in a wavelength range greater than 300nm, sharp absorption peaks at 354nm and 335nm, a shoulder at 324nm...
Embodiment 3
[0107] Embodiment 3: the synthesis of borate 2 (wherein R 3 = n-octyl)
[0108] (1) 1-(2-bromo-5-octylphenyl)pyrene
[0109] 1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene (3.28g, 0.01mol) and 1,2-dibromo-4-octane Phenylbenzene (6.96g, 0.02mol) was placed in a 100mL flask with a stirring bar in advance, vacuumed, and nitrogen gas was passed three times, then oxygen-free toluene (40mL) was injected into the flask with a syringe. The mixture was heated to 80°C and stirred until the monomers were completely dissolved, and tetrakis(triphenylphosphide)palladium (Pd(PPh 3 ) 4 ) (0.01mmol) in toluene (10mL), and inject anaerobic nitrogen saturated 2M sodium carbonate solution (10mL) with a syringe. Then the system was warmed up to 90°C. After 48 hours of reaction, the temperature of the system was lowered to room temperature, and saturated sodium bicarbonate solution was added to separate the liquids. The organic phase was washed three times with dichloromethane. After the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com