Novel synthetic method of loratadine
A newly synthesized technology for loratadine, which is applied in the field of preparation of loratadine, can solve the problems of high risk, high impurity content, and many pollutants in the process, and achieve stable process, good repeatability, and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
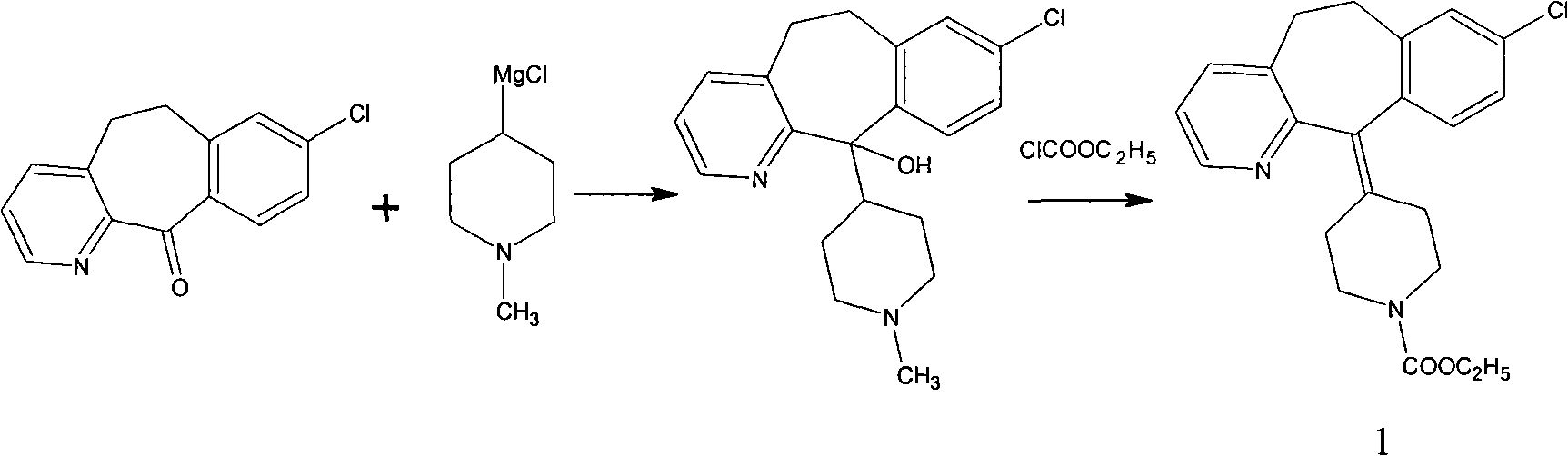
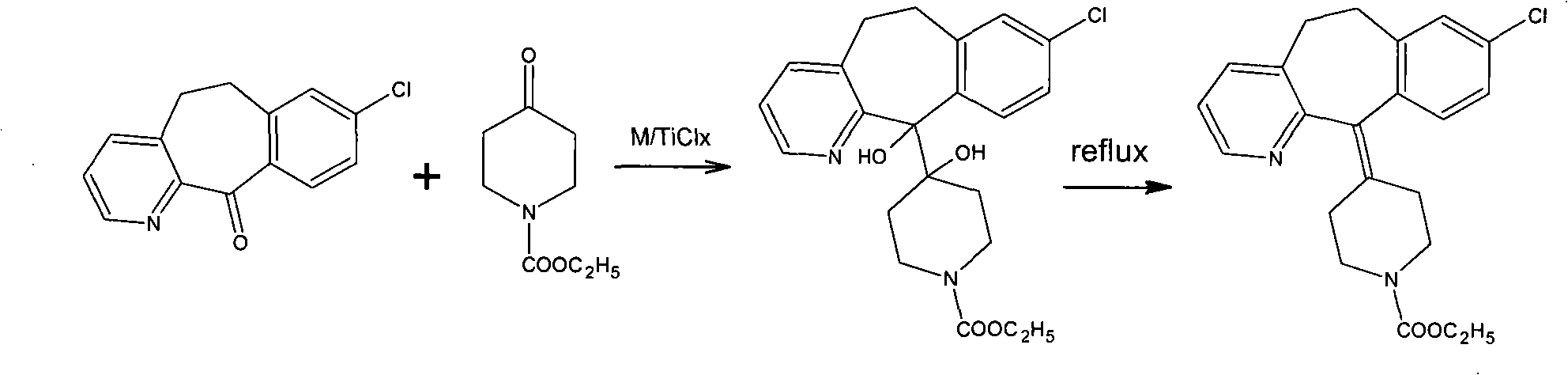
[0017] Dissolve 10 g of tricyclic ketone and 7.4 g of 4-ethoxycarbonylpiperidone in 50 ml of n-hexane solution and set aside.
[0018] Put 6.6g of magnesium powder and 120ml of n-hexane into a 250ml flask; stir and control the temperature to 10°C; add 12ml of titanium tetrachloride dropwise and control the dropwise addition for 30 minutes; keep warm at 10°C for 30 minutes; add n-hexane solution dropwise for 30 minutes Add dropwise; finish, keep warm for 2 hours; keep warm, add 2 drops of concentrated sulfuric acid, heat up and reflux for 3 hours; finish, add 100g of water, stir for 30 minutes; separate liquid, add 40ml of n-hexane to extract the water layer twice; combine the oil layer , washed twice with 50ml of water; after that, 150ml of n-hexane was concentrated, cooled to room temperature and crystallized to obtain the crude product of loratadine. Recrystallized from acetonitrile to obtain 11 g of white solid. The yield is 70%, and the melting point is 132-136°C.
Embodiment 2
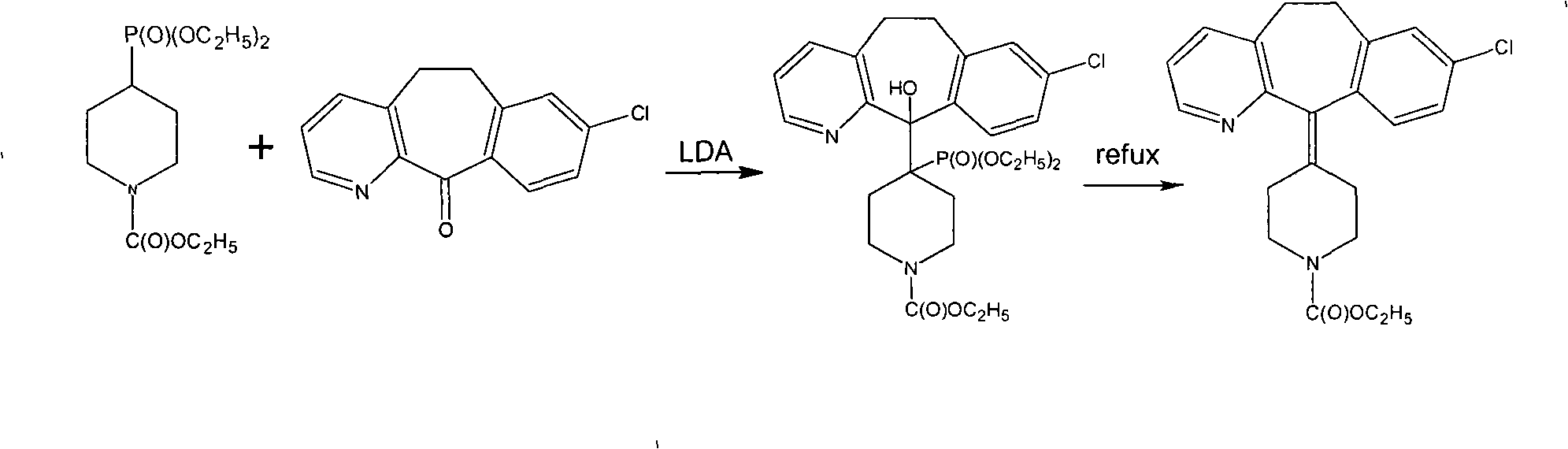
[0020] 10 g of tricyclic ketone and 7.4 g of 4-ethoxycarbonylpiperidone were dissolved in 40 ml of toluene solution and set aside.
[0021] Put 7.4g of aluminum powder and 120ml of toluene into a 250ml flask; stir and control the temperature to 20°C; add 12ml of titanium tetrachloride dropwise and control the dropwise addition for 30 minutes; keep warm at 20°C for 30 minutes; add the toluene solution dropwise for 30 minutes OK; Bi, keep warm for 2 hours; keep warm well, add 2 drops of concentrated sulfuric acid, heat up and reflux for 3 hours; finish, add 100g of water, stir for 30 minutes; separate liquid, add 40ml of toluene to extract the water layer twice; combine the oil layers, use 50ml Washed twice with water; after that, toluene was recovered to dryness, and acetonitrile was added to cool down to room temperature for crystallization to obtain crude loratadine. Recrystallized from acetonitrile to obtain 11.2 g of white solid. The yield is 71.3%, and the melting point i...
Embodiment 3
[0023] 10 g of tricyclic ketone and 7.4 g of 4-ethoxycarbonylpiperidone were dissolved in 40 ml of methyl tetrahydrofuran solution and set aside.
[0024] Put 6.6g of magnesium powder and 120ml of methyl tetrahydrofuran into a 250ml flask; stir and control the temperature to 0°C; add 12ml of titanium tetrachloride dropwise, and add dropwise for 30 minutes; keep warm at 0°C for 30 minutes; add methyltetrahydrofuran solution dropwise Add dropwise in 30 minutes; finish, keep warm for 2 hours; keep warm, add 2 drops of concentrated sulfuric acid, heat and reflux for 3 hours; finish, recover methyl tetrahydrofuran to dryness; add water 100g and petroleum ether, stir for 30 minutes; separate liquid, water The layer was extracted twice by adding 40ml of petroleum ether; the combined oil layers were washed twice with 50ml of water; after that, 150ml of petroleum ether was concentrated, cooled to room temperature for crystallization, and crude loratadine was obtained. Recrystallized fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com