Hydrophilic medicament dual-microsphere formulation and preparation method thereof
A technology of hydrophilic drugs and microspheres, which can be used in pharmaceutical formulations, medical preparations of non-active ingredients, powder delivery, etc. Rapid, increased drug loading, high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
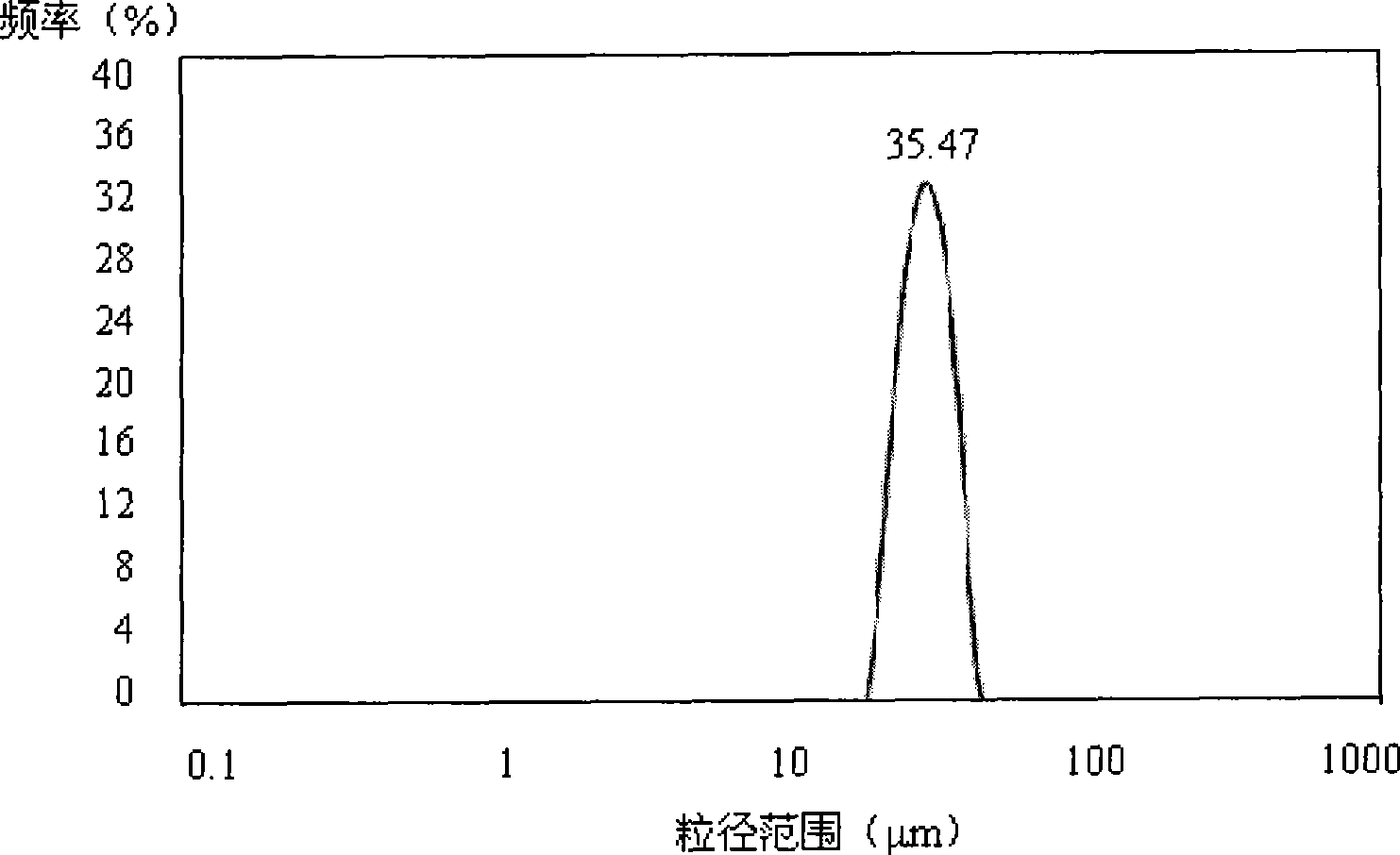
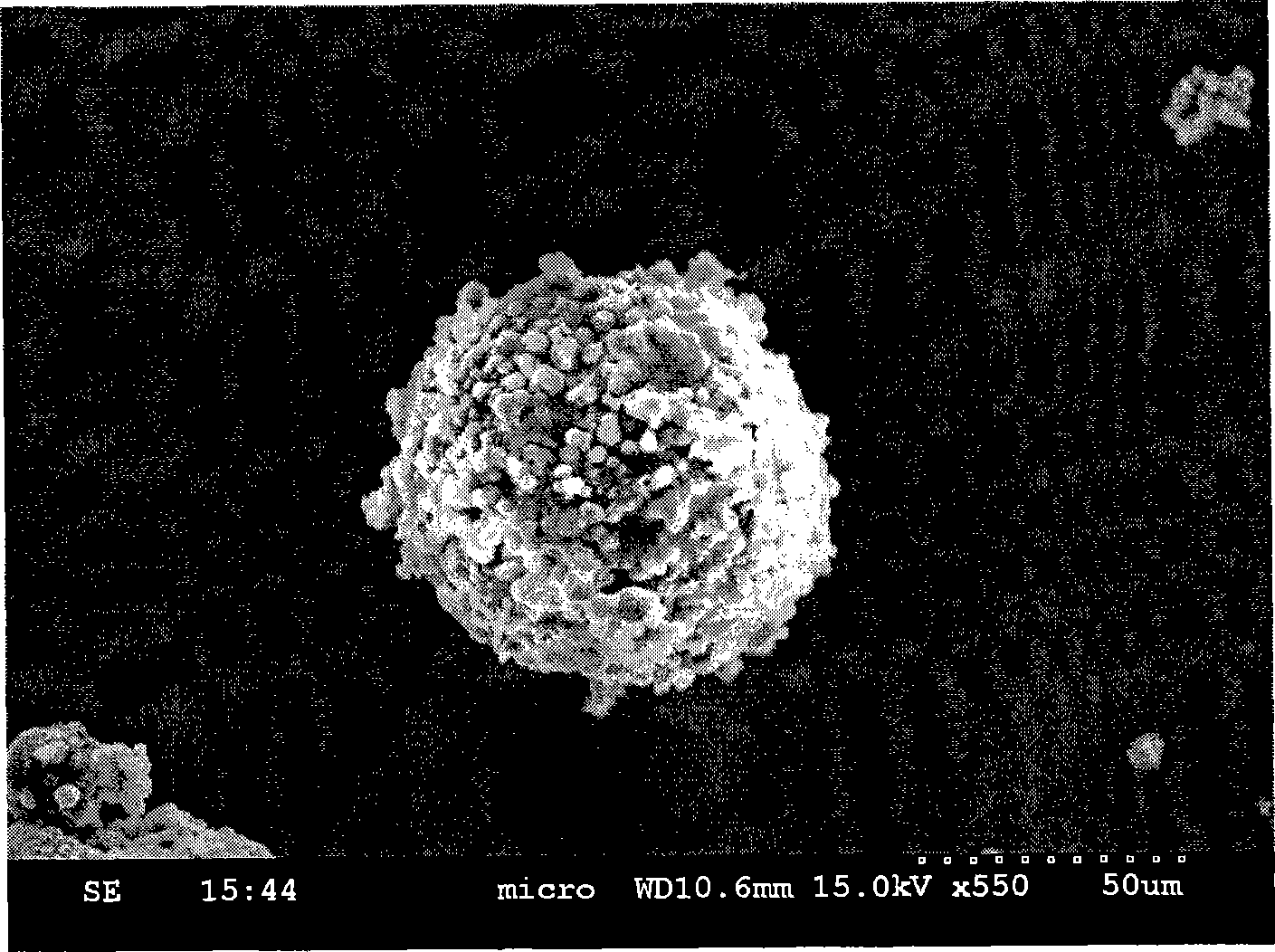
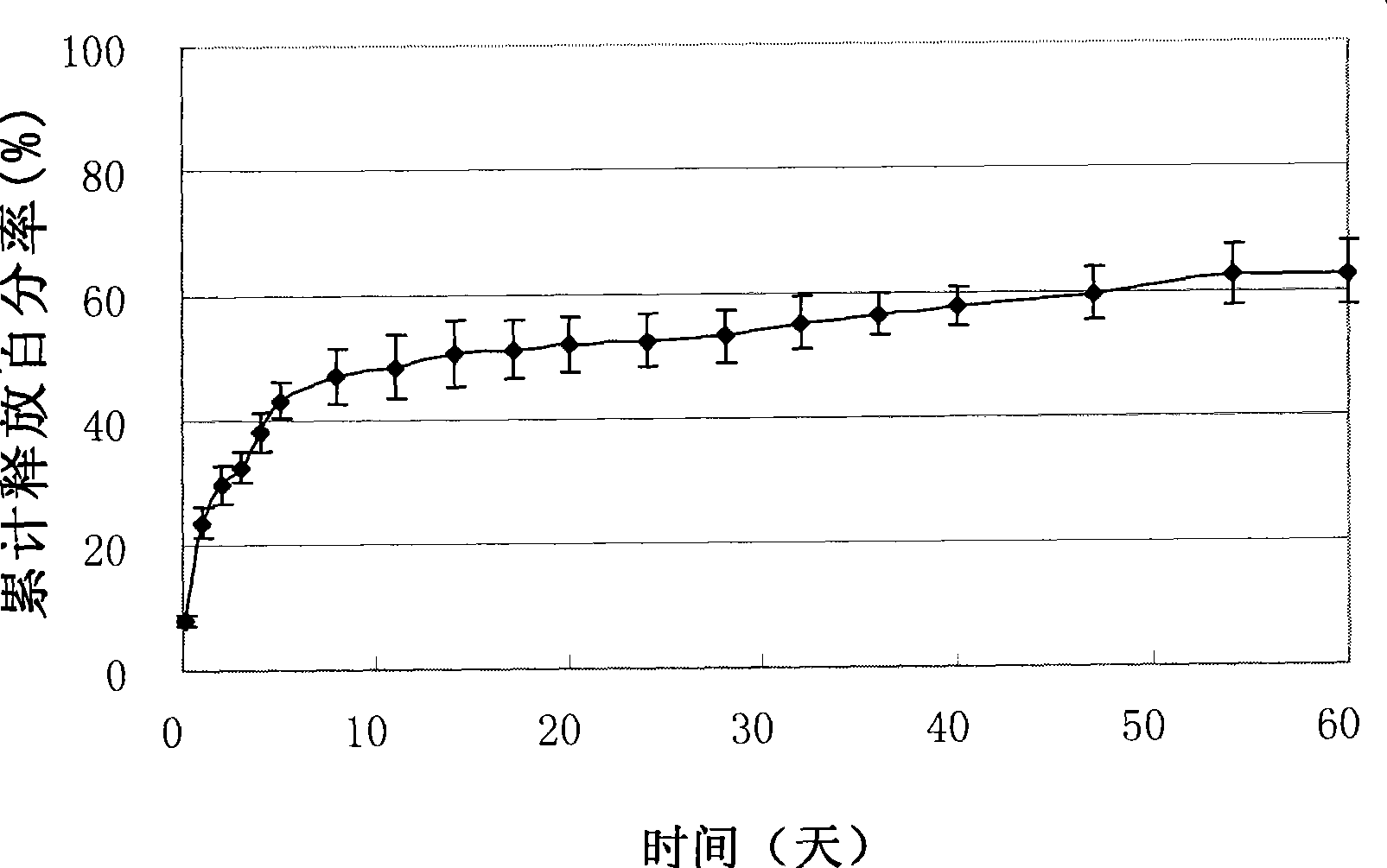
Image
Examples
Embodiment 1
[0037]Accurately weigh 30 mg of gentamicin, dissolve in 30 ml of chitosan aqueous solution with a concentration of 1 mg / mL and pH=6, stir magnetically at room temperature, slowly add 12 ml of sodium tripolyphosphate aqueous solution with a concentration of 0.5 mg / mL into chitosan The sugar solution was mixed for 30 minutes, and the precipitate was collected after centrifugation at 12000 rpm at 4°C for 20 minutes, washed 3 times with distilled water, and freeze-dried to obtain chitosan nanoparticles.
[0038] Disperse the above dried 50mg chitosan nanoparticles into 2.5ml acetonitrile solution containing 300mgPLGA (the mass ratio of LA to GA is 90:10), mix evenly with 400w probe type ultrasonic instrument for 90 seconds, and use it as the suspension water phase Dissolve 1.5g Span 80 in 20ml edible oil as the oil phase; slowly drop the oil phase into the above-mentioned suspension water phase under stirring at 3500rpm, rotate and evaporate under reduced pressure for 2 hours, remo...
Embodiment 2
[0040] Accurately weighed 30mg streptomycin, and the rest were operated in the same way as in Example 1.
Embodiment 3
[0042] Accurately weigh 250 mg of tetracycline, dissolve in 25 ml of chitosan aqueous solution with a concentration of 3 mg / mL and pH=4, stir magnetically at room temperature, slowly add 20 ml of aqueous sodium tripolyphosphate solution with a concentration of 1.5 mg / mL to the chitosan solution, and mix After 40 minutes, centrifuge at 16,000 rpm at 4°C for 20 minutes to collect the precipitate, wash with distilled water three times, and freeze-dry to obtain chitosan nanoparticles.
[0043] The above dried 50mg chitosan nanoparticles were dispersed into 3ml of acetonitrile solution containing 200mgPLGA (the mass ratio of LA and GA was 85:15), and the 400w probe type ultrasonic instrument was ultrasonically mixed for 90 seconds as the suspension water phase; Dissolve 2.0g of Span 80 in 20ml of edible oil as the oil phase. Slowly drop the oil phase into the above suspension water phase under stirring at 3500rpm. Rotate and evaporate under reduced pressure for 1.5 hours to remove a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com