Method for producing polyurethane and use of polyurethane produced by the same
A manufacturing method and polyurethane technology, applied in the field of polyurethane, can solve the problems of inability to increase the molecular weight of the elastomer, difficult to remove the solvent, insufficient solubility, etc., and achieve the effects of excellent dyeability and mechanical properties, high dissolution speed, and small residual deformation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
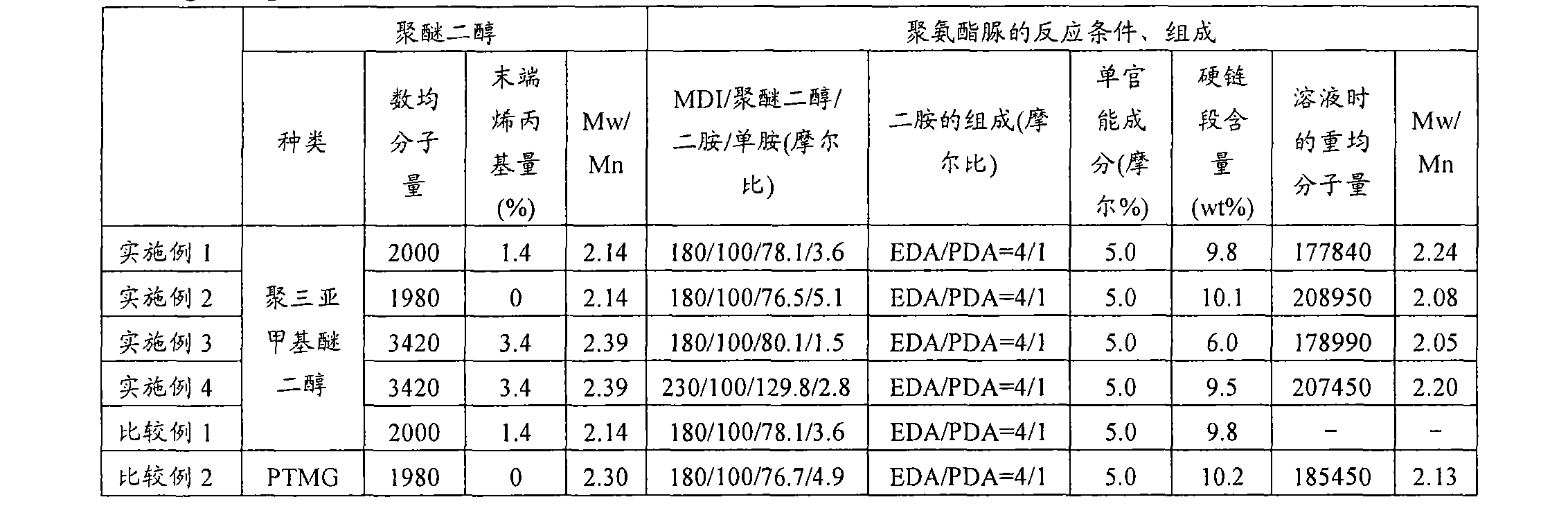
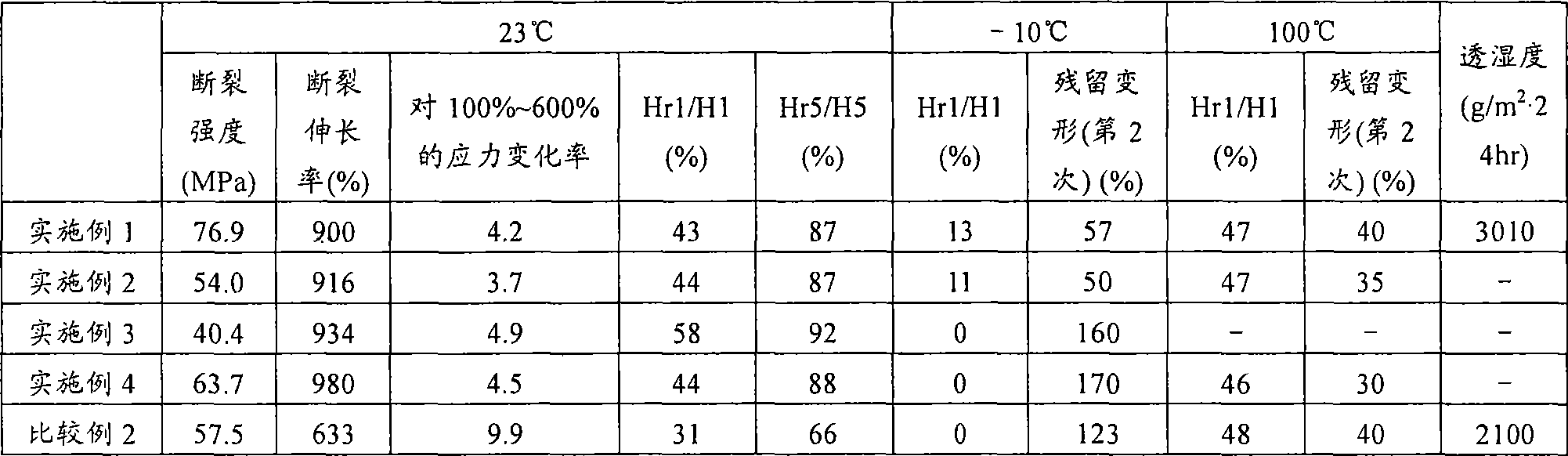
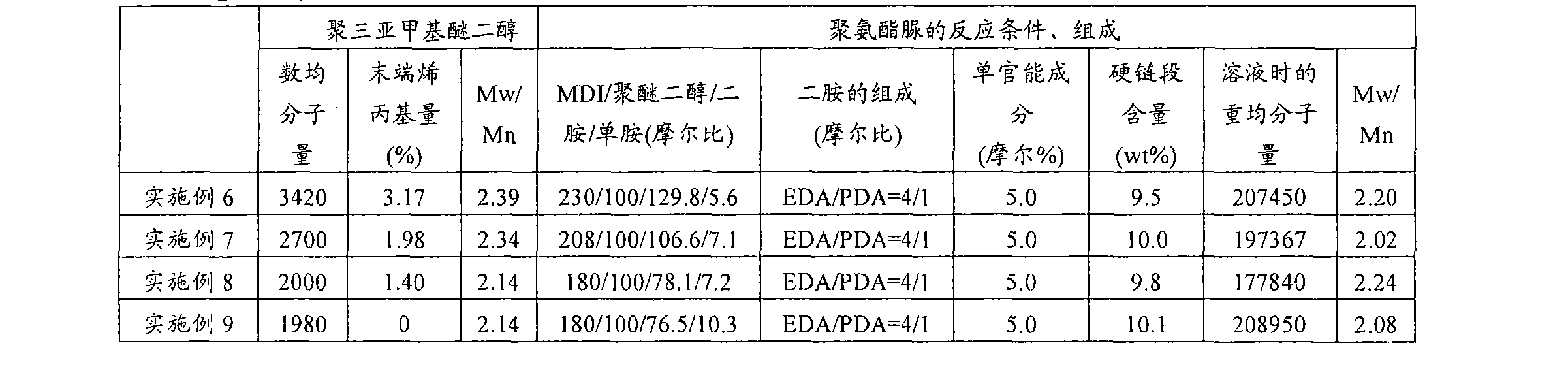
[0179] Hereinafter, although the Example of this invention is demonstrated more concretely, this invention is not limited to these Examples unless the summary is exceeded. In addition, analysis and measurement in the following examples and comparative examples were performed in accordance with the following methods.
[0180]
[0181] The number average molecular weight of polytrimethylene ether glycol was calculated|required from the hydroxyl value (KOH (mg) / g).
[0182]
[0183] Utilization of terminal allyl groups in polytrimethylene ether glycol 1 H-NMR ("AVANCE400" manufactured by BRUKER) was measured.
[0184]
[0185] The molecular weight distribution of polyether polyol was measured as follows: A tetrahydrofuran solution of polyether polyol was prepared, and a gel permeation chromatography (GOC) device [manufactured by Tosoh Corporation, product name "HLC-8220" (column: TSKgelSuperHZM-N(3 root))], a standard curve was prepared using a tetrahydrofuran detection k...
reference example 1
[0209] Manufacture of Polytrimethylene Ether Glycol
[0210]
[0211] Into a 1000 mL four-necked flask equipped with a distillation tube, a nitrogen introduction tube, a mercury thermometer, and a stirrer, 500 g of 1,3-propanediol was added while nitrogen was supplied at 1 liter / min. After adding 0.348 g of sodium carbonate there, while stirring, 6.78 g of 95% by weight concentrated sulfuric acid was slowly added. The flask was heated by immersing it in an oil bath, and the temperature of the liquid in the flask reached 163° C. over about 1.5 hours. The time when the liquid temperature in the flask reached 163° C. was set as the reaction start point, and then, the liquid temperature was kept at 163° C., and the reaction was carried out for 18 hours. Water produced by the reaction was distilled off along with nitrogen.
[0212] The reaction solution naturally cooled to room temperature was transferred to a 2 L four-necked flask containing 500 g of desalted water, and it was...
Embodiment 1
[0216] 2200.84 g of polytrimethylene ether glycol to which 5 ppm phosphoric acid was added (the number average molecular weight calculated from the hydroxyl value is 2000, and the terminal allyl group ratio is 1.4%), which was preheated to 40°C, was placed in a 3 L separable flask. , followed by the addition of 499.16 g of diphenylmethane diisocyanate (MDI) (NCO / OH ratio = 1.80) warmed to 40°C. Installed in a 45° C. oil bath, the temperature of the oil bath was raised to 70° C. over 1 hour while stirring with an anchor stirring blade (150 rpm) under a nitrogen flow, and then kept at 70° C. for 3 hours. After confirming that the reaction rate of NCO exceeded 98% by titration, the prepolymer was transferred to a 2 L tin can, and kept overnight in a thermostat at 40°C.
[0217] 1,848 g of prepolymer and 2,772 g of dehydrated dimethylacetamide (DMAC, manufactured by Kanto Chemical Co., Ltd.) were placed in a prepolymer tank, stirred and dissolved at room temperature, and then cool...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com