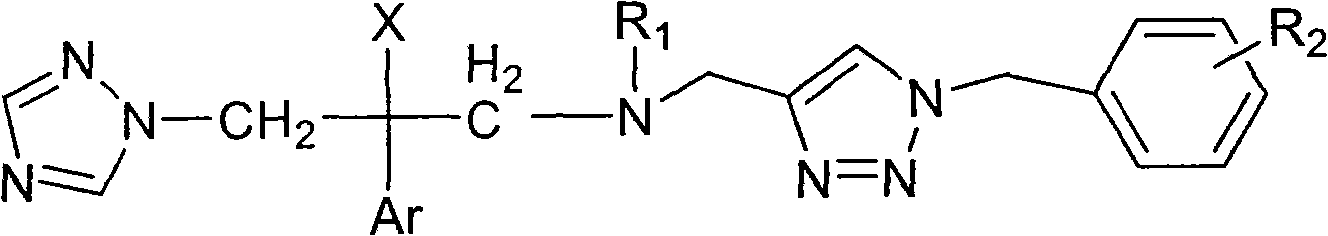
Novel azole antifungal compound and preparation method thereof
A technology for antifungal drugs and compounds, applied in the field of medicine, to achieve the effects of high yield, good antifungal effect and broad antifungal spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of intermediate 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-alkylamino-2- alcohol
[0048] (1) Preparation of 2-chloro-2', 4'-difluoroacetophenone
[0049] Put 200g (1.494mol) of anhydrous aluminum trichloride and 150g (1.30mol) of m-difluorobenzene into a 1000mL three-necked flask, stir at room temperature, and slowly add 150g (1.30mol) of chloroacetyl chloride dropwise. Continue to stir at room temperature for 30 minutes, slowly raise the temperature to 45°C, continue to stir at this temperature for 4.5 hours, pour the reaction solution into ice water as usual, precipitate a solid, and filter; the filtrate is extracted twice with 800 mL of dichloromethane, The dichloromethane extracts were combined, washed with water until neutral, dried over anhydrous sodium sulfate, filtered, and the solvent was recovered to obtain a solid, which was combined twice and recrystallized with ethanol to obtain 2-chloro 2',4'-difluorophenethyl Ketone 21...
Embodiment 2
[0061] Example 2: Preparation of intermediate 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-alkyl-N-yne Propylamino)-2-ol
[0062] (1) Preparation of 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-isopropyl-N-propargyl) Amino)-2-ol
[0063] Add 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-isopropylamino)- 1.7g (5mmol) of 2-alcohol, 1.2g (10mmol) of propyne bromide, 2.76g (20mmol) of anhydrous sodium carbonate, 20mL of acetonitrile, heated to reflux in an oil bath for 8 hours, then evaporated to dryness, extracted with 30mL of ethyl acetate, and filtered , ethyl acetate column chromatography [developing agent chloroform:methanol (V / V, the same below) 60:1], finally get 1-(1H-1,2,4-triazol-1-yl)-2-( 1.30 g of 2,4-difluorophenyl)-3-(N-isopropyl-N-propargylamino)-2-ol, yield 62.2%.
[0064] (2) Preparation of 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-n-propyl-N-propargyl) Amino)-2-ol
[0065] Add 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluoroph...
Embodiment 3
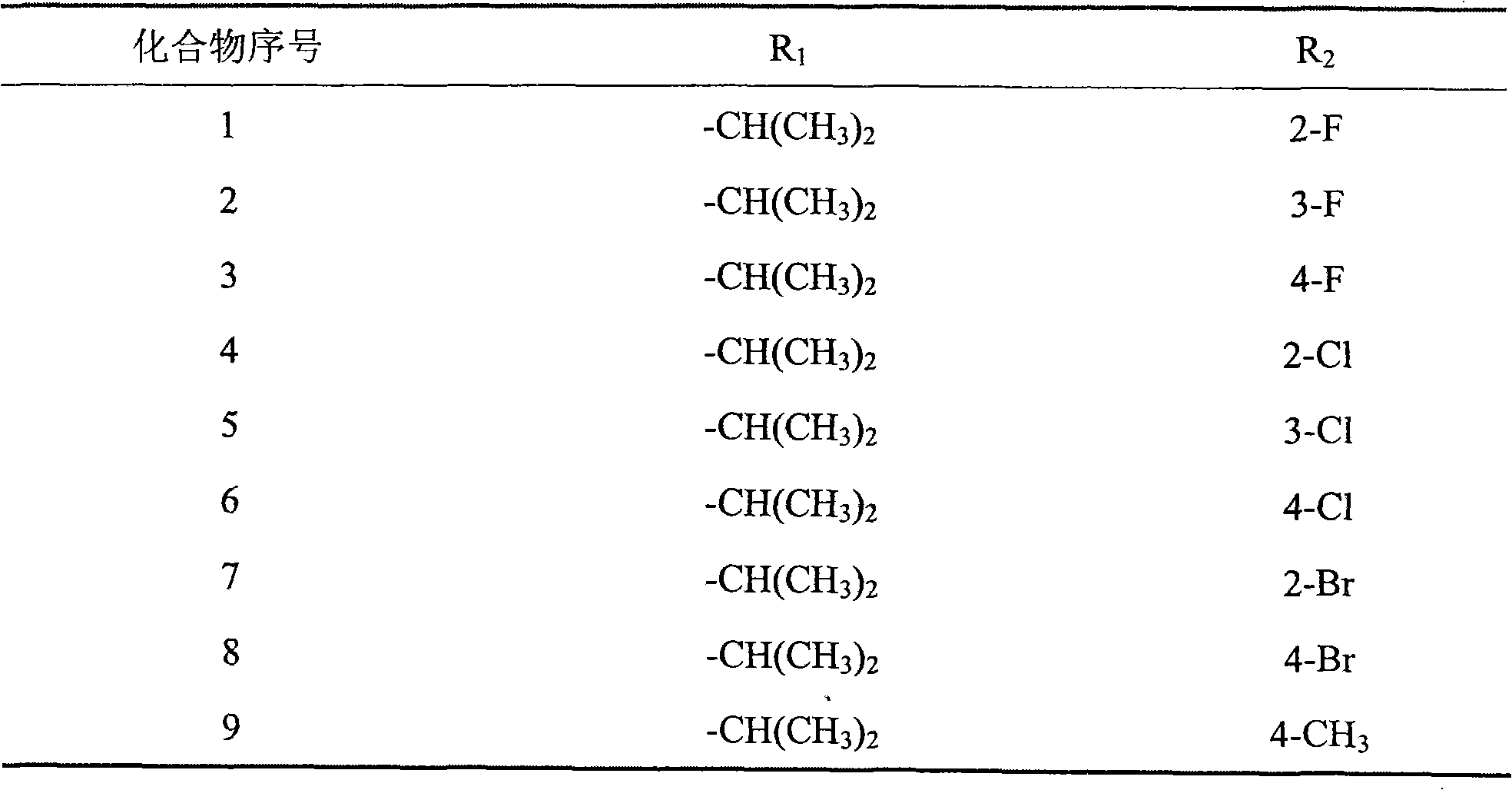
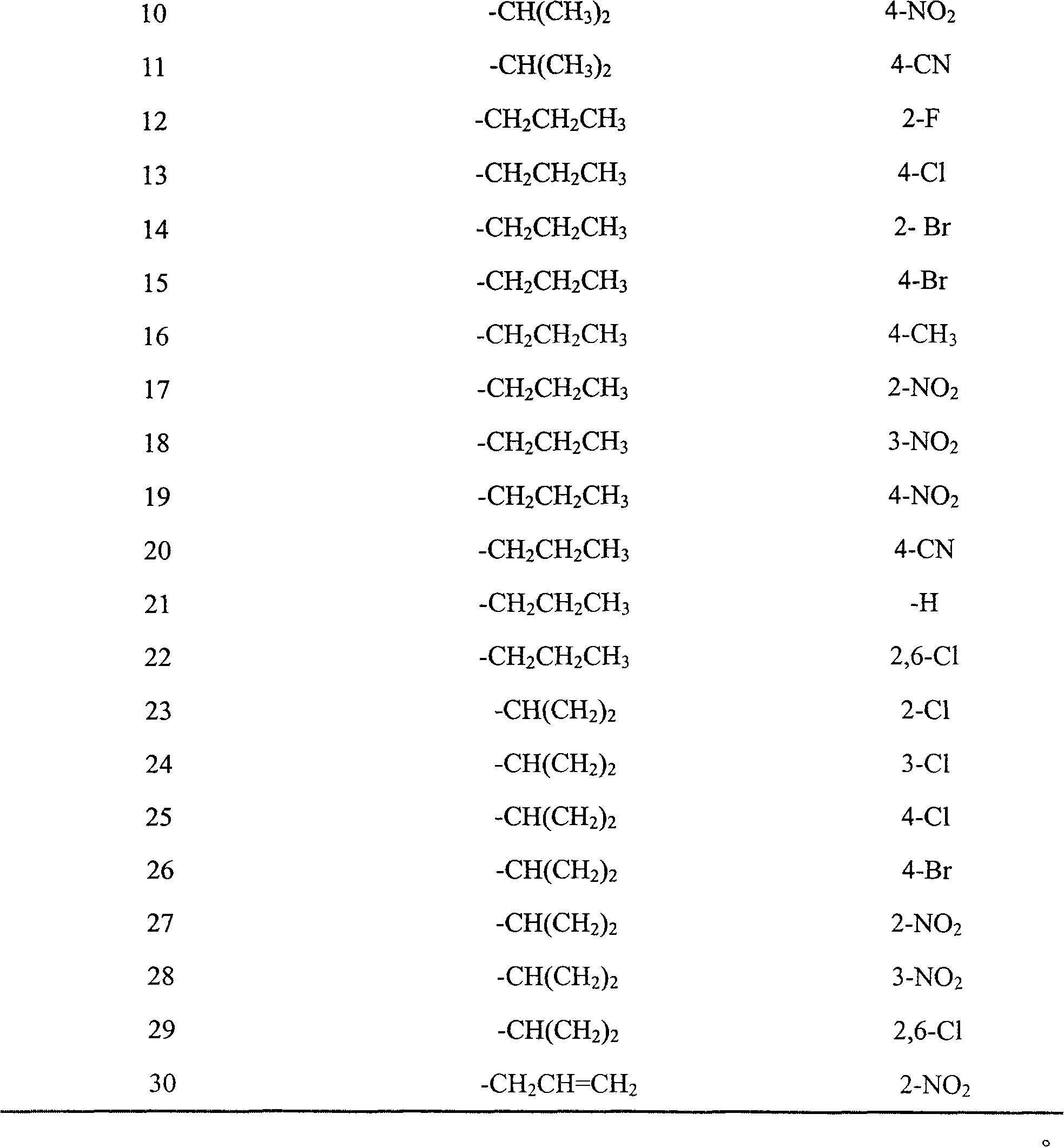
[0070] Example 3: Preparation of 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-isopropyl-N-[1H -1,2,3-triazol-4-yl-1-(2-fluorobenzyl)methylamino]}-2-alcohol (compound 1 in Table 1)
[0071] Add 100mg (1.4mmol) of sodium azide, 200mg (1.2mmol) of o-fluorobenzyl bromide, and 15mL of dimethyl sulfoxide into a 25mL eggplant-shaped flask, and react with magnetic stirring at room temperature for 6h. Then add 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-isopropyl-N-propargylamino) -2-ol 200mg (0.6mmol), sodium ascorbate 20mg, CuSO 4 ·5H 2 O 25mg, water 1mL, stirred and reacted at room temperature for 10min, poured the reaction solution into dilute ammonia water, extracted with ethyl acetate (20mL×2), acidified the ethyl acetate layer with dilute hydrochloric acid (20mL×2), separated the water layer and the water layer use Na 2 CO 3 Adjust the pH to about 7, extract with ethyl acetate (20mL×2), dry the ethyl acetate layer with anhydrous sodium sulfate for 4h, fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com