Cydiodine buccal tablets and preparation method thereof
A technology of cydidinium iodine and mass percentage, applied in the field of cydidinium lozenges and their preparation, can solve the problems of increasing the intake of sucrose, physical harm and the like, and achieve the effects of reducing the amount of sucrose and reducing potential harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
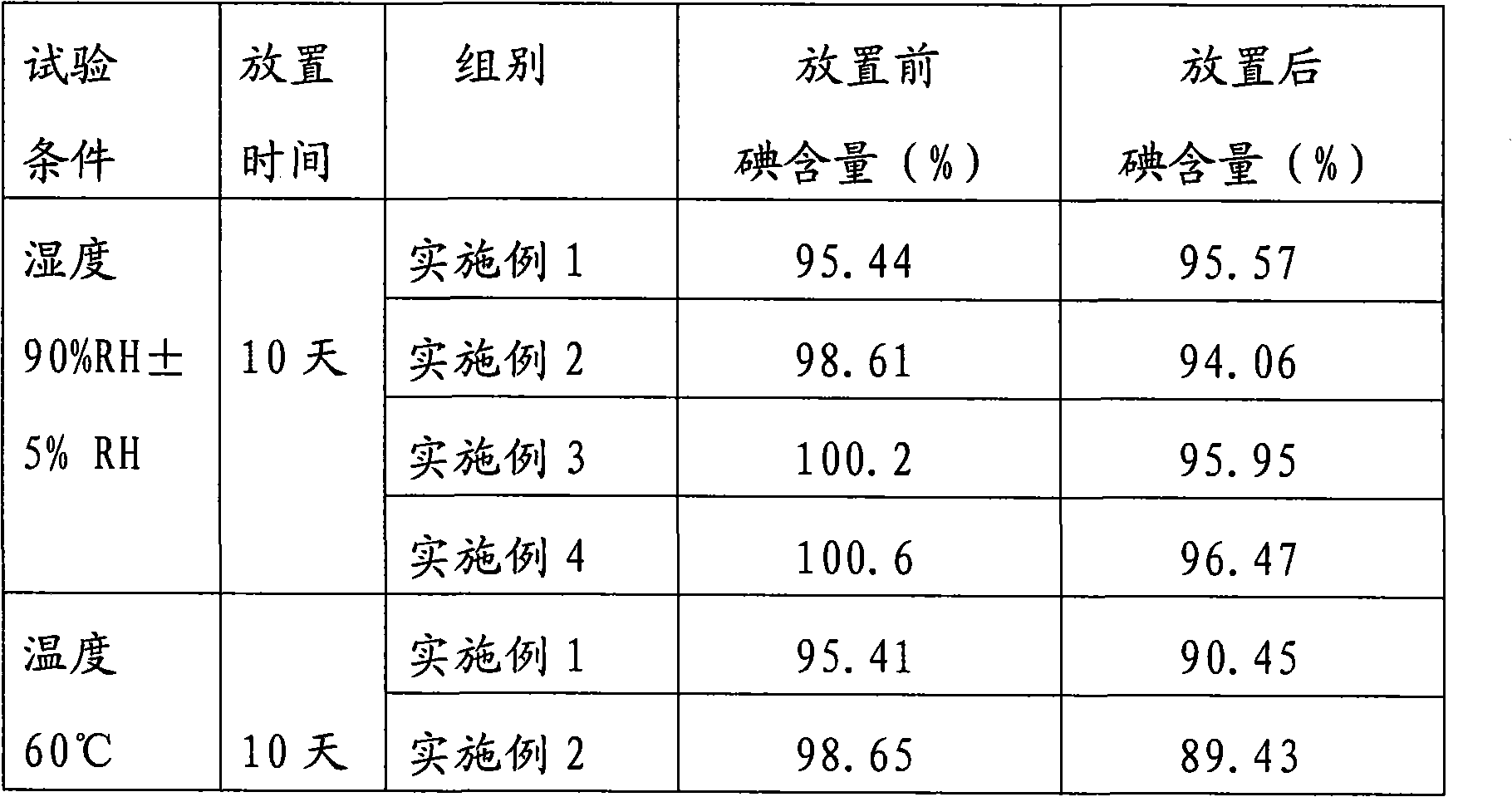
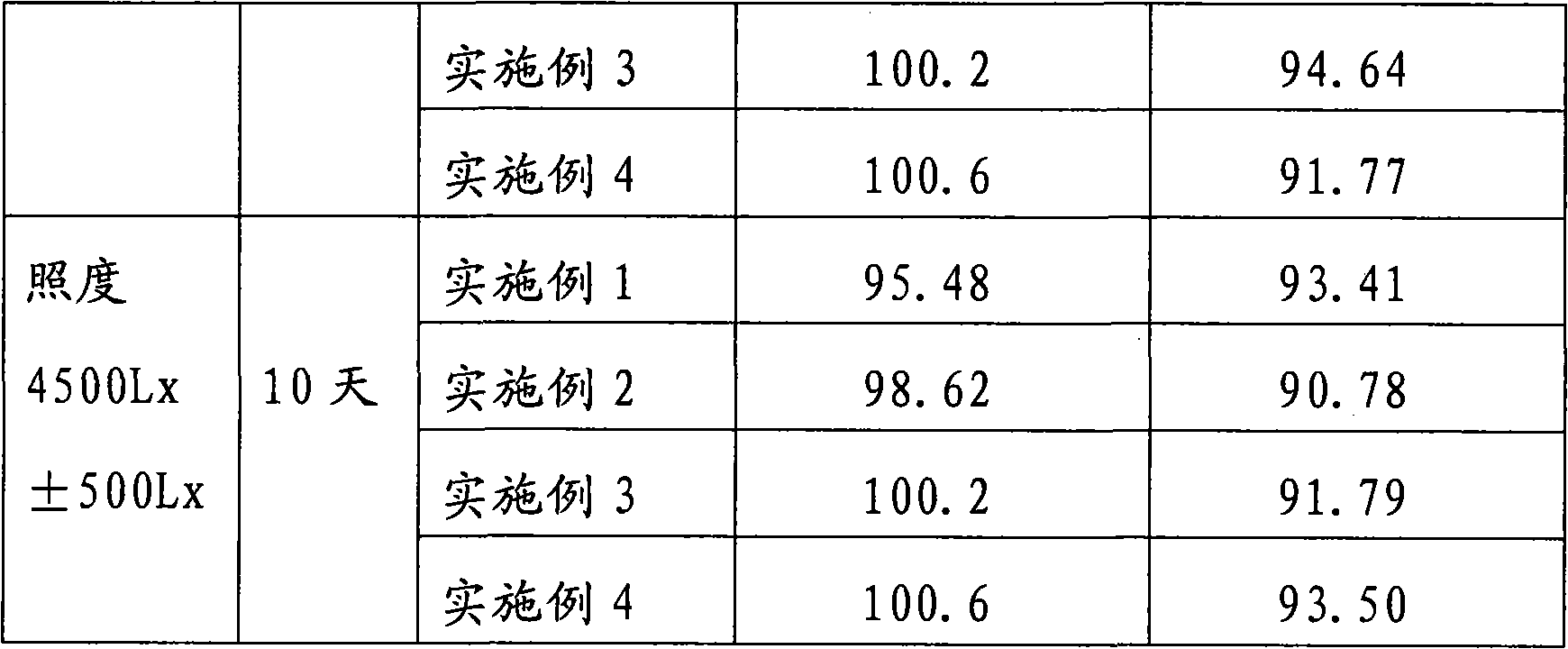
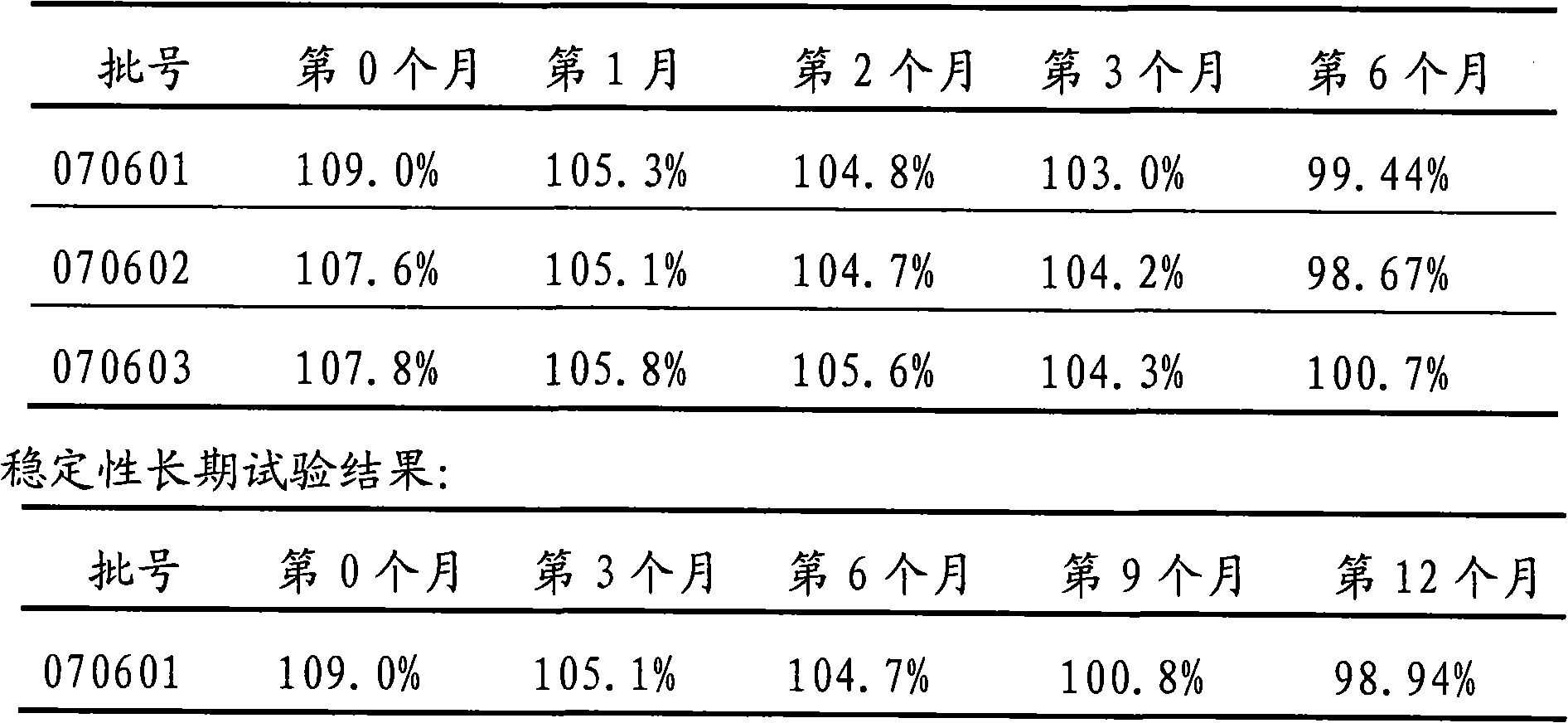
Embodiment 1
[0052] The components and weight of cediodine buccal tablets are:
[0053] 8 g of cedi-iodine, 18 g of cedithiophene, 400 g of lactose, 4 g of magnesium stearate, and a 3% HPMC (hydroxypropyl methylcellulose) aqueous solution with a mass percent concentration.
[0054] Among them, the composition and weight of Sidi iodine are: 6.97g of iodine, 5.06g of potassium iodide, and 35g of β-cyclodextrin. The components and weight of cedibranch are: menthol 5.5g, β-cyclodextrin 55g.
[0055] The preparation method of cediodine buccal tablets:
[0056] 1) Preparation of cediiodine and cedichol: the cediiodine and cedicephalus are prepared by cyclodextrin inclusion technology, which is a saturated aqueous solution method.
[0057] 2) After uniformly mixing cediodine and lactose according to the amount of the components in an equal increment method, adding an appropriate amount of 3% HPMC aqueous solution by mass percentage to make a soft material;
[0058] 3) making the soft material ...
Embodiment 2
[0063] The components and weight of cediodine buccal tablets are:
[0064] 8 g of cedi iodine, 400 g of mannitol, 18 g of cedithiophene, 2 g of aspartame, 4 g of magnesium stearate, and the mass percentage concentration is 3% HPMC aqueous solution.
[0065] Wherein, the composition and weight of cediiodine and cediole are the same as in Example 1.
[0066] The preparation method of cediodine buccal tablets:
[0067] 1) same as the 1st step of embodiment 1;
[0068] 2) After mixing the cediodine and mannitol uniformly in an equal increment method according to the component amount, add an appropriate amount of 3% HPMC aqueous solution to make a soft material;
[0069] 3) same as the 3rd step of embodiment 1;
[0070]4) Weighing cedinail, aspartame and magnesium stearate according to the component amounts, and mixing them uniformly with the dried granules;
[0071] 5) The following steps are the same as the 5th and 6th steps of Example 1.
Embodiment 3
[0073] The components and weight of cediodine buccal tablets are:
[0074] Sidi iodine 8g, xylitol 80g, cedipol 18g, isomalt 320g, magnesium stearate 4g.
[0075] Wherein, the composition and weight of cediiodine and cediole are the same as in Example 1.
[0076] The preparation method of cediodine buccal tablets:
[0077] 1) same as the 1st step of embodiment 1;
[0078] 2) each component is weighed by amount and screened with a 60 mesh sieve;
[0079] 3) Mix the screened 60-mesh components evenly with equal increment method;
[0080] 4) The following steps are the same as the 5th and 6th steps of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com