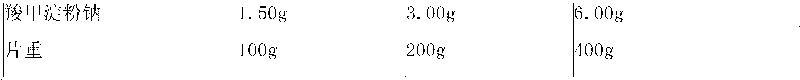Medicinal composition of calcium-containing antagonist, A II receptor antagonist and statins
A receptor antagonist, calcium ion antagonist technology, applied in the field of medicine, can solve the problem of unsatisfactory blood pressure effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
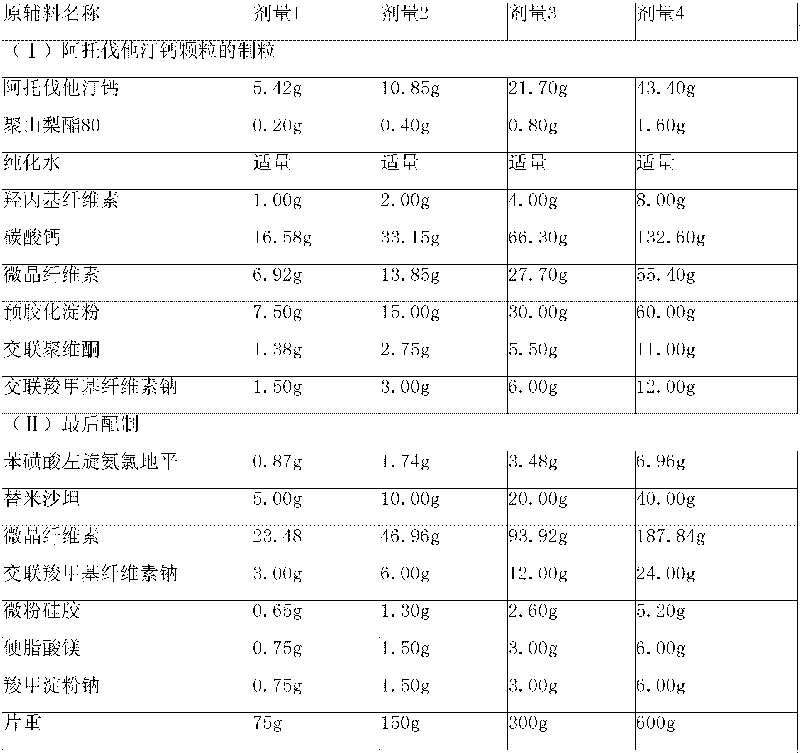
[0162] Embodiment 1: levamlodipine besylate, telmisartan and atorvastatin calcium tablet
[0163]
[0164] Preparation:
[0165] (1) Granulation of atorvastatin calcium granules
[0166] Step 1. Pass various solid raw and auxiliary materials through No. 5 to No. 6 sieves, and set aside;
[0167] Step 2, dissolving polysorbate 80 in purified water at 45°C to 60°C, adding hydroxypropyl cellulose, and cooling the solution to room temperature;
[0168] Step 3, mixing atorvastatin calcium, calcium carbonate, microcrystalline cellulose, precrosslinked starch, crospovidone and croscarmellose sodium in a granulator;
[0169] Step 4. Mix the powder mixture from step 3 and the solution from step 2 in the granulator, and stir while adding to make a suitable soft material. If necessary, adjust its pH to 5.5-10.0. Use No. 2 Sieve to make wet granules;
[0170] Step 5, dry the granules in the drying equipment, sieve the granules with No. 2 sieve after drying, and finally make the moi...
Embodiment 2
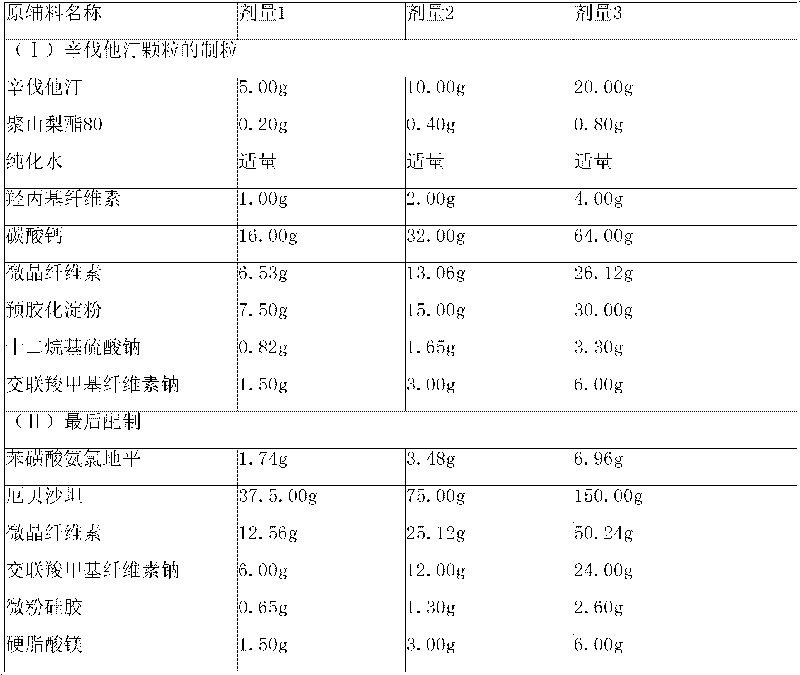
[0176] Embodiment 2: amlodipine besylate, irbesartan and simvastatin capsules
[0177]
[0178]
[0179] Preparation:
[0180] (1) Granulation of Simvastatin Granules
[0181] Step 1. Pass various solid raw and auxiliary materials through No. 5 to No. 6 sieves, and set aside;
[0182] Step 2, dissolving polysorbate 80 in purified water at 50°C and adding hydroxypropyl cellulose, and cooling the solution to room temperature;
[0183] Step 3, mixing simvastatin, calcium carbonate, microcrystalline cellulose, precrossified starch, sodium lauryl sulfate and croscarmellose sodium in a granulator;
[0184] Step 4. Mix the powder mixture from step 3 and the solution from step 2 in the granulator, and stir while adding to make a suitable soft material, and adjust its pH value to 5.5-10.0 if necessary; use No. 2 Sieve to make wet granules;
[0185] Step 5, dry the granules in the drying equipment, sieve the granules with No. 2 sieve after drying, and finally make the moisture...
Embodiment 3
[0191] Embodiment 3: levamlodipine besylate, losartan potassium and atorvastatin calcium dispersible tablet
[0192]
[0193]
[0194] Preparation:
[0195] (1) Preparation of Atorvastatin Calcium Microcapsules
[0196] Step A, pass various solid raw and auxiliary materials through No. 5 to No. 6 sieves respectively, and set aside;
[0197] Step B. Dissolve gelatin and gum arabic in purified water respectively, stir to make them fully dissolved, add atorvastatin calcium and cross-linked carboxymethyl cellulose sodium to gum arabic, ultrasonic emulsify for 45 minutes, and gelatin solution and Mix the gum arabic solution into a three-necked flask, control the stirring speed at 200-400rpm, heat in a water bath, keep the temperature at 45°C-50°C, adjust the pH value of the system to 3.5-4.0, conduct the condensation reaction for 55 minutes, and lower the temperature of the system to 2 ℃-8℃, add formaldehyde with a mass concentration of 25% and a glutaraldehyde solution wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com