Lansoprazole sodium suspension preparation for injection and new application thereof
A technology of lansoprazole sodium and suspension preparations, applied in the field of preparation of lansoprazole sodium suspension preparations for injection, can solve the problems of high insoluble particles, easy oxidative degradation, easy precipitation and crystallization, etc. Low cost, low price, good resolubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
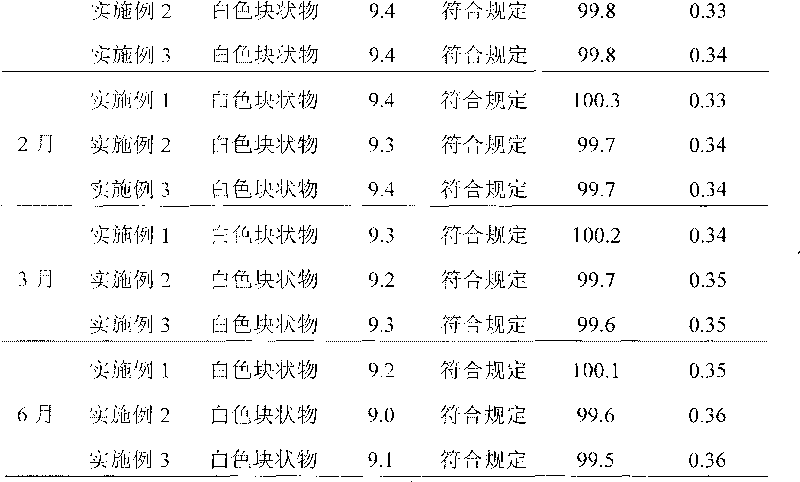
Embodiment 1
[0048] The preparation of embodiment 1 injection lansoprazole sodium suspension
[0049] Prescription (specification 30mg)
[0050] Lansoprazole Sodium 30g
[0051] Polysorbate 80 7.5g
[0052] Poloxamer 188 15g
[0053] Cellulose acetate 15g
[0054] Butylated Hydroxyanisole 7.5g
[0056] Preparation Process:
[0057] A. 7.5g polysorbate 80, 15g poloxamer 188 and 30g lansoprazole sodium are dissolved in 500ml water for injection to form an aqueous phase;
[0058] B. Stir and disperse 15g of cellulose acetate and 7.5g of butyl hydroxyanisole in 50ml of dichloromethane to form an organic phase;
[0059] C. Add the organic phase to the water phase while stirring, then transfer it to a high-pressure homogenizer, fully stir and emulsify for 2 hours, and obtain an emulsion;
[0060] D. add 22.5g sodium chloride in this emulsion, fully stir and mix, carry out sub-packaging in the vial, freeze-drying, obtained the finished product of lansoprazol...
Embodiment 2
[0073] The preparation of embodiment 2 injection lansoprazole sodium suspension
[0074] Prescription (specification 15mg)
[0075] Lansoprazole Sodium 15g
[0076] Glyceryl Stearate 5g
[0077] Poloxamer 188 10g
[0078] Ethylcellulose 15g
[0079] Vitamin E 7.5g
[0080] Mannitol 15g
[0081] Preparation Process:
[0082] A. 5g glyceryl stearate, 10g poloxamer 188 and 15g lansoprazole sodium are dissolved in 700ml water for injection to form an aqueous phase;
[0083] B. Stir and disperse 15g of ethyl cellulose and 7.5g of vitamin E in 70ml of ethyl acetate to form an organic phase;
[0084] C. Add the organic phase to the water phase while stirring, then transfer it to a high-pressure homogenizer, fully stir and emulsify for 2 hours, and obtain an emulsion;
[0085] D. Add 15g mannitol in this emulsion, fully stir and mix, carry out subpackage in the vial, lyophilize, obtained the finished product of lansoprazole sodium suspension preparation.
Embodiment 3
[0098] The preparation of embodiment 3 injection lansoprazole sodium suspension
[0099] Prescription (specification 30mg)
[0100] Lansoprazole Sodium 30g
[0101] Poloxamer 188 30g
[0102] Glyceryl Stearate 7.5g
[0103] Ethylcellulose 19g
[0104] Vitamin E 10.5g
[0105] Lactose 27g
[0106] Preparation Process:
[0107]A. 7.5g glyceryl stearate, 30g poloxamer 188 and 30g lansoprazole sodium are dissolved in 1000ml water for injection to form an aqueous phase;
[0108] B. Stir and disperse 19g ethylcellulose and 10.5g vitamin E in 100ml ethyl acetate to form an organic phase;
[0109] C. Under stirring, add the organic phase to the water phase, then transfer it to a high-pressure homogenizer, fully stir and emulsify for 2.5 hours, and obtain an emulsion;
[0110] D. Add .27g lactose in this emulsion, fully stir and mix, carry out sub-package in the vial, lyophilize, obtained the finished product of lansoprazole sodium suspension preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com