Magneto-optical dual-mode molecular image probe and preparation method thereof
A molecular imaging and dual-mode technology, which is applied in the fields of nuclear magnetic resonance/magnetic resonance imaging contrast agents, pharmaceutical formulations, preparations for in vivo tests, etc., can solve the problem of imperfect molecular imaging technology, inability to provide clinical diagnosis, low resolution, etc. problems, to achieve the effects of excellent thermodynamic stability, excellent photostability, and high quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of Ir-Gd complexes: Take the compound phenylpyridine-o-phenanthroline-1,4,7-tri(tert-butyl ester)-1,4,7,10-tetraazacyclododecane iridium complex 74.7mg (0.05mmol) was placed in a three-necked flask, N 2Add 2mL of dichloromethane under the atmosphere, then add 1mL of trifluoroacetic acid dropwise therein, stir overnight, track the plate until the end of the reaction, spin off the solvent and unreacted acid to obtain a brownish-yellow oil, and then take 22.55mg (0.05 mmol)Gd(NO 3 ) 3 .6H 2 O was dissolved in 1.5mL of water, added to the oil, adjusted to pH 7 with 1M NaOH, stirred for 24 hours, filtered with suction to obtain a brownish-yellow solid, and purified by reverse-phase HPLC to obtain a yellow powder. MS (ESI + ) m / z: 1237.3 ([M+H] + ).
Embodiment 2
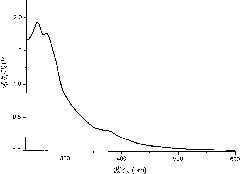
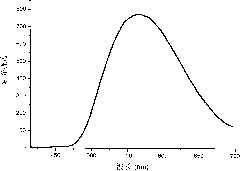
[0022] UV-visible spectrum and phosphorescence spectrum test of Ir-Gd complex: UV-visible and phosphorescence spectrum tests are all carried out in aqueous solution, wherein phosphorescence spectrum is excited by 405nm ultraviolet light, the test results are shown in figure 1 with figure 2 .
Embodiment 3
[0024] Cell fluorescence confocal imaging experiments of Ir-Gd complexes: KB cells were provided by the Chinese Institute of Biochemistry and Cell Biology. The cells were cultured in medium (MEM) containing 10% fetal bovine serum (FBs) at 37°C in 5% CO 2 in progress. The cells were plated for 24 hours, and the cell concentration was 5×10 8 / L. The confocal imaging of the cell imaging experiment was completed under an OLYMPUS FV1000 laser scanning microscope and a 60x objective lens. A 405nm semiconductor laser is used to excite the phosphorescence emission of the Ir-Gd complex, and then the emission with a wavelength ranging from 520nm to 620nm is collected. Before the experiment, the cells were washed with PBS buffer solution, and then incubated with PBS buffer solution of Ir-Gd complex (16.7 μM) at 37°C for 30 minutes; after that, the cell imaging experiment was performed after washing with PBS buffer solution. see results image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com