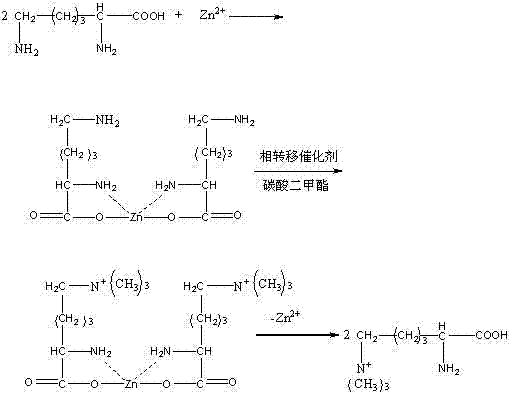Method for preparing laminine and pharmaceutically acceptable salts thereof
A technology for laminin and medicinal salts, which is applied in the field of preparation of laminin and its medicinal salts, can solve the problems of increased product, incomplete reaction, and reduced product yield, achieving high yield and low cost , good quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Preparation L-lysine zinc
[0032] Add 36.4 kg of L-lysine hydrochloride into 50 liters of deionized water, heat to 65° C. to dissolve it, and adjust the pH value to 7.2 with ammonia water. 13.64 kg of zinc chloride was added to 40 liters of deionized water to dissolve. Add zinc chloride solution dropwise to L-lysine hydrochloride aqueous solution while stirring, and keep warm for 110 min. Suction filtration while hot at 83°C, heat and concentrate the filtrate until a crystal film appears, cool, grow crystals for 24 hours, a large number of crystals are precipitated, filter with suction, wash the filter cake twice with absolute ethanol, recrystallize twice with ethanol aqueous solution, and use Wash once with absolute ethanol to obtain the finished product of L-lysine zinc chelate, without drying, and set aside. The molar yield of the obtained product relative to L-lysine is 87%, and the purity of the zinc L-lysine product is 99.5%.
Embodiment 2
[0033] Embodiment 2 The preparation of laminin crude product
[0034] In the reaction vessel equipped with stirrer, thermometer and constant pressure funnel, add 15 liters of deionized water, add 10 kg of L-lysine zinc prepared in Example 1, dissolve, add tributylbenzyl chloride under stirring Ammonium chloride 0.2kg. Then add ammonia water to adjust the pH to 8.3, alternately add dimethyl carbonate, the total amount of dimethyl carbonate is 2.5 times (molar ratio) of L-lysine zinc, and react at room temperature for 4.5 hours. Leave to stand for stratification, separate the organic phase, and recover the dimethyl carbonate from the organic phase for mechanical use. Sodium hydrosulfide was added to the aqueous phase, stirred for 2 hours, and the insoluble matter was removed by filtration. The filtrate was concentrated under reduced pressure, and ethanol was added until a crystal film appeared, crystallized in an ice bath, grown for 2 hours, separated and dried to obtain cru...
Embodiment 3
[0035] Embodiment 3 The preparation of laminin hydrochloride crude product
[0036] In the reaction vessel equipped with stirrer, thermometer and constant pressure funnel, add 13 liters of deionized water, add 10 kg of L-lysine zinc obtained in Example 1, dissolve, add tributylbenzyl chloride under stirring Ammonium chloride 0.25kg. Then add ammonia water to adjust the pH to 7.9, alternately add dimethyl carbonate, the total amount of dimethyl carbonate is 2.5 times (molar ratio) of L-lysine zinc, and react at room temperature for 4.2 hours. Leave to stand for stratification, separate the organic phase, and recover the dimethyl carbonate from the organic phase for mechanical use. Sodium hydrosulfide was added to the aqueous phase, stirred for 2 hours, and the insoluble matter was removed by filtration. The filtrate was concentrated under reduced pressure, the pH was adjusted to 6.9 with hydrochloric acid, and ethanol was added until a crystal film appeared, crystallized in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com