Mixed manganese ferrite catalysts, method of preparing thereof and method of preparing 1,3-butadiene using thereof
A manganese ferrite catalyst, mixed technology, applied in the direction of catalyst activation/preparation, carbon compound catalyst, heterogeneous catalyst chemical elements, etc., can solve the problems of excess, lower yield, lower economic efficiency, etc., to achieve simple structure and Effects of synthesis steps, high reproducibility, and economic efficiency improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
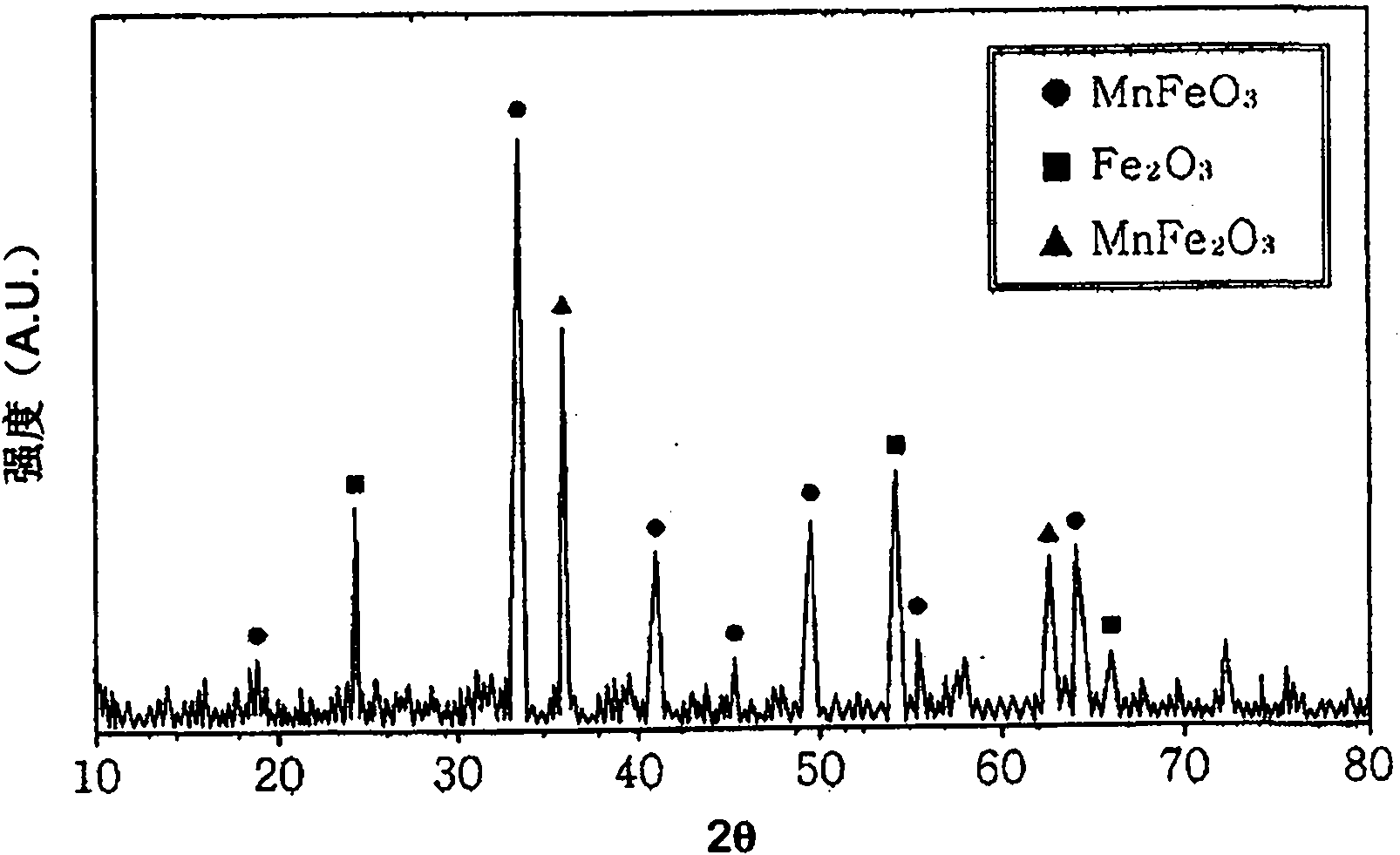
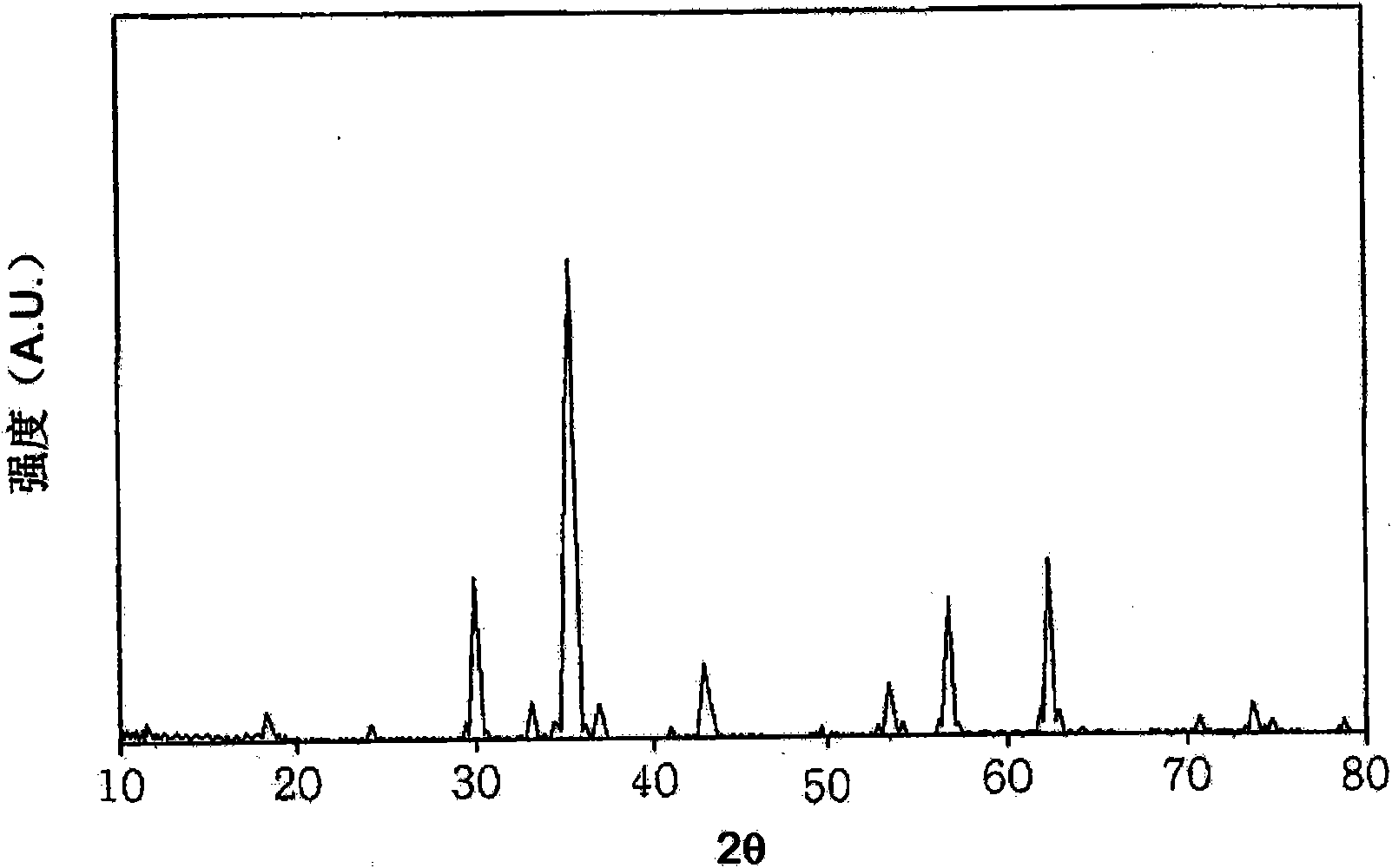
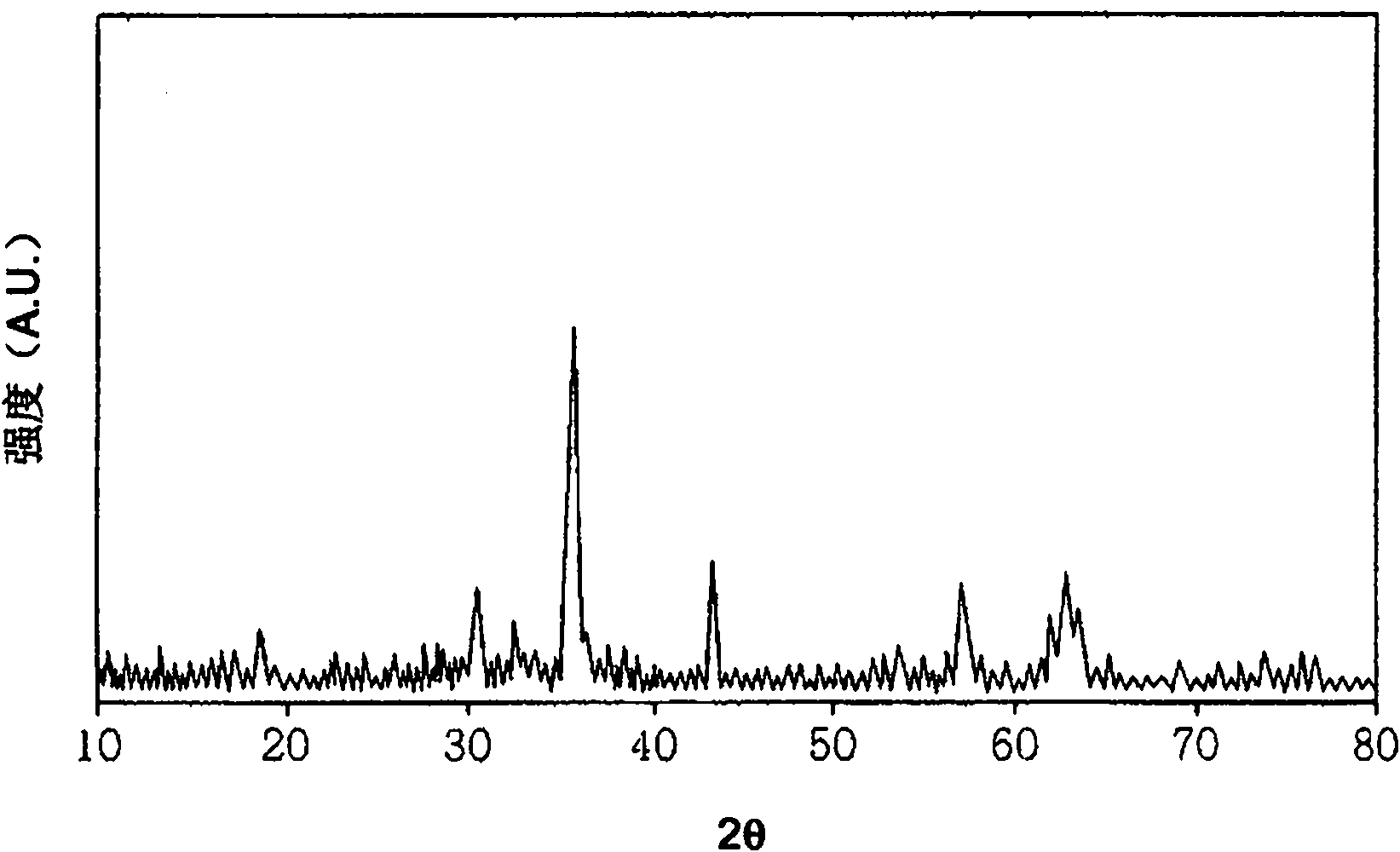
[0034] According to Preparation Example 1 of the present invention, by X-ray diffraction analysis, the phase characteristics of the catalyst samples prepared by co-precipitation at room temperature were compared, and it was found that the catalyst samples containing iron oxide (α-Fe 2 o 3 ) and ferromanganese oxide (MnFeO 3 ) of mixed manganese ferrite, rather than single-phase manganese ferrite (see figure 1 ). In contrast to this, in the case of each catalyst prepared in Preparation Examples 2 and 3, it was found that single-phase zinc ferrite and single-phase manganese ferrite were formed (see figure 2 with 3 ).
[0035] Therefore, the catalyst for preparing 1,3-butadiene of the present invention is a mixed type manganese ferrite catalyst which can be conveniently prepared at room temperature without additional pretreatment and posttreatment, and its Has high regenerative properties.
[0036] In X-ray diffraction analysis, the mixed manganese ferrite catalyst of the ...
preparation example 1
[0047] Preparation of Mixed Manganese Ferrite Catalyst
[0048] In order to prepare the mixed manganese ferrite catalyst, manganese dichloride tetrahydrate (MnCl 2 4H 2 O) as the manganese precursor and using ferric chloride hexahydrate (FeCl 3 ·6H 2 O) as iron precursors. Both the zinc precursor and the iron precursor are materials that are readily soluble in distilled water. 198 g of manganese dichloride tetrahydrate and 541 g of iron trichloride hexahydrate were dissolved in distilled water (1000 ml) and mixed with each other, followed by thorough stirring to form an aqueous precursor solution. Next, after confirming that the precursor had been completely dissolved in distilled water, the aqueous precursor solution was added dropwise at a constant rate to an aqueous sodium hydroxide solution (6000 ml) having a concentration of 3M to form a mixed solution. The mixed solution was well stirred at room temperature for 12 hours with a magnetic stirrer, and then left at ro...
preparation example 2
[0063] Preparation of Zinc Ferrite Catalyst
[0064] Adopt the same method as preparation example 1 to prepare single-phase zinc ferrite catalyst, the difference is: do not use manganese precursor, instead, use 136g zinc chloride (ZnCl 2 ) as a zinc precursor. Depend on figure 2 It can be seen from the X-ray diffraction analysis that the catalyst prepared in Preparation Example 2 is a single-phase zinc ferrite catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com