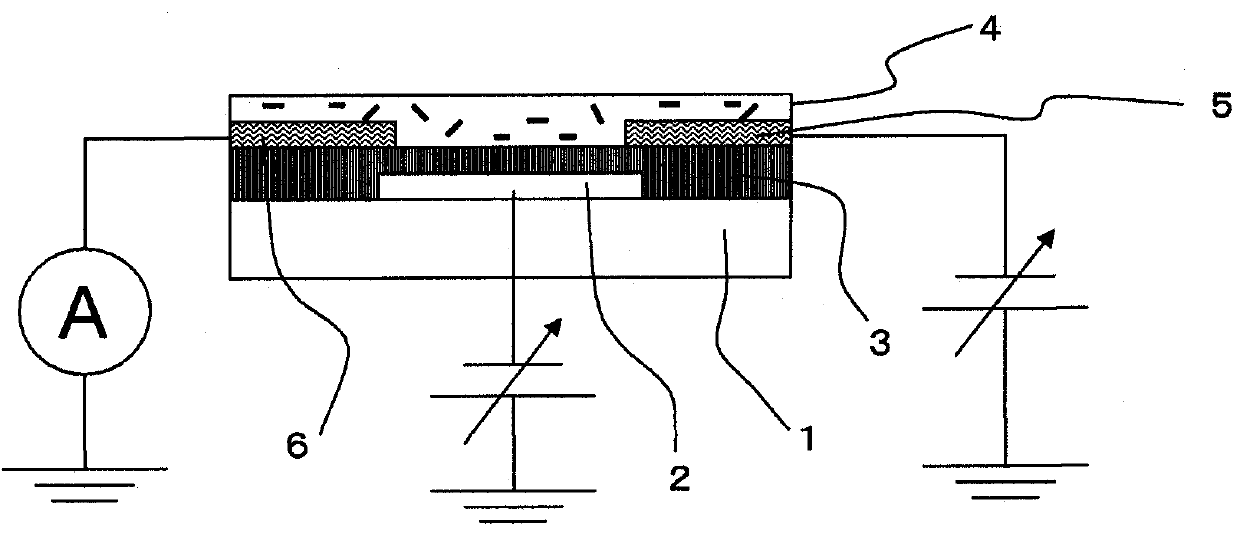
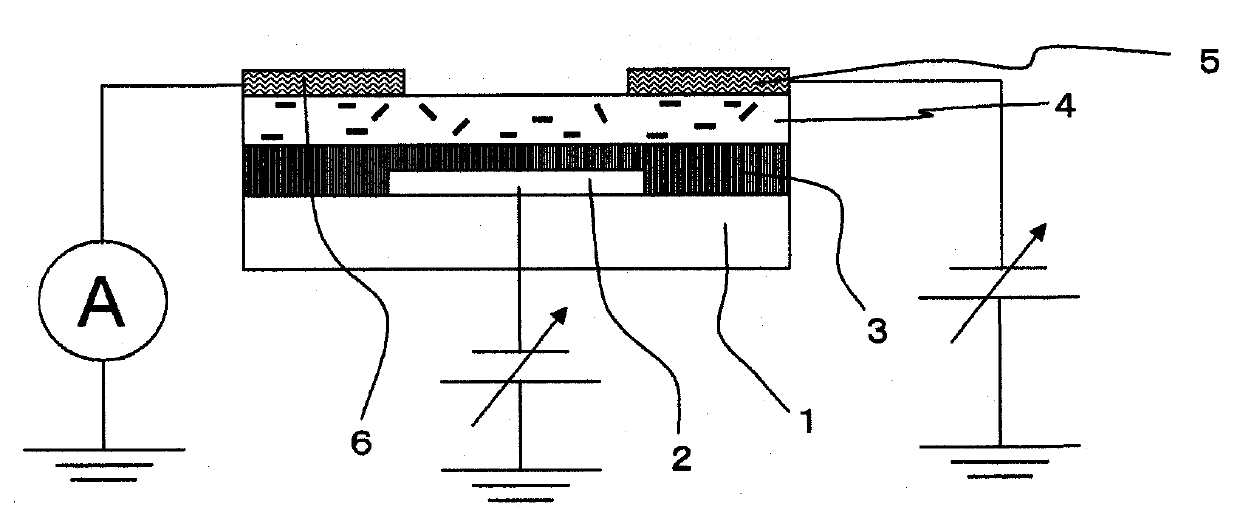
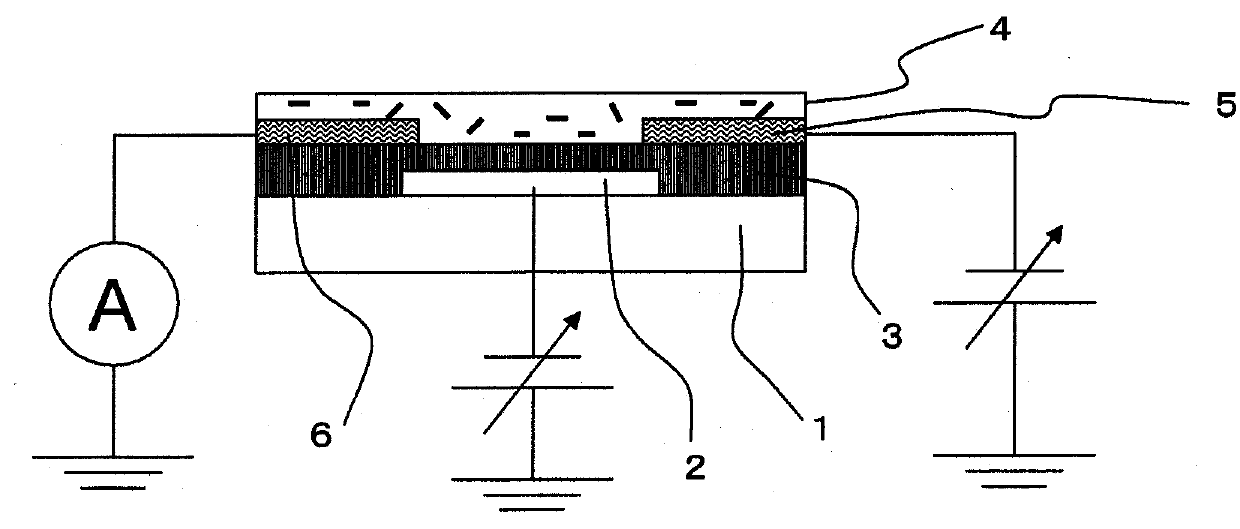
Carbon nanotube composite, organic semiconductor composite, and field-effect transistor
A technology of field-effect transistors and organic semiconductors, which is applied in the manufacture of transistors, semiconductor devices, semiconductor/solid-state devices, etc., can solve problems such as large hysteresis, and achieve the effect of reduced hysteresis and high mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0111] and, in 1 An FT-NMR apparatus (JEOLJNM-EX270 manufactured by JEOL Ltd.) was used for the H-NMR measurement.
[0112] In addition, the average molecular weight (number average molecular weight, weight average molecular weight) was measured using a GPC apparatus (manufactured by TOSOH Corporation, high-speed GPC apparatus HLC-8220GPC), using chloroform as a mobile phase. Using a calibration curve obtained by measuring the relationship between elution time and molecular weight using polystyrene standard samples, the average molecular weight of the sample was calculated by the absolute calibration curve method. The degree of polymerization n was calculated by the following formula.
[0113] Degree of polymerization n=[(weight average molecular weight) / (molecular weight of 1 monomer unit)].
[0114] Synthesis Example 1 (Synthesis of Compound [21])
Synthetic example 1
[0115] Compound [21] was synthesized by the method shown in Formula 1.
[0116] 【chemical 11】
[0117]
[0118] 4.3 g of compound (1-a) (manufactured by Tokyo Chemical Industry Co., Ltd.) and 10 g of bromine (manufactured by Wako Pure Chemical Industries, Ltd.) were added to 150 ml of 48% hydrobromic acid, and stirred at 120° C. for 3 hours. After cooling to room temperature, the precipitated solid was filtered with a glass filter, and washed with 1000 ml of water and 100 ml of acetone. The obtained solid was vacuum-dried at 60° C. to obtain 6.72 g of compound (1-b).
[0119] Dissolve 10.2 g of compound (1-c) in 100 ml of dimethylformamide, add 9.24 g of N-bromosuccinimide (manufactured by Wako Pure Chemical Industries, Ltd.), and stir at room temperature for 3 Hour. 200 ml of water, 200 ml of n-hexane and 200 ml of dichloromethane were added to the obtained solution, and the organic layer was separated. The obtained organic layer was washed with 200 ml of water, and dr...
Synthetic example 2
[0128] Compound [22] was synthesized by the method shown in Formula 2.
[0129] 【Chemical 12】
[0130]
[0131] In 1,4-two Add 0.34g of compound (2-a), 0.85g of bis-valeryl diboron, and 0.86g of potassium acetate to 7ml of alkane, and then add 0.21g of [bis(diphenylphosphino)ferrocene]palladium dichloride under nitrogen atmosphere , stirred at 80°C for 9 hours. 100 ml of water and 100 ml of ethyl acetate were added to the obtained solution, and the organic layer was separated. The obtained organic layer was washed with 100 ml of water, and dried over magnesium sulfate. The resulting solution was purified by column chromatography (filler: silica gel, eluent: dichloromethane) to obtain 167 mg of compound (2-b).
[0132] 110 mg of the above compound (1-i) and 17 mg of the above compound (2-b) were dissolved in 6 ml of toluene. 2 ml of water, 0.22 g of potassium carbonate, 9 mg of tetrakis(triphenylphosphine)palladium(0) and 1 drop of Aliquat(R)336 (manufactured by Aldrich...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| magnetic hysteresis | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com