Supported arsine catalyst as well as synthesizing method and application thereof in Wittig reaction
A catalyst and application technology, applied in the field of supported arsine catalyst, synthesis and its application in Wittig reaction, to achieve the effect of convenient synthesis and homogeneous reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Sodium acetate (0.369g, 4.5mmol) was added to a three-neck flask equipped with a pumping head and a constant pressure dropping funnel, and diketene (7.56g, 90mmol) and acetonitrile (50.0mL), ice salt The temperature of the bath was cooled to -5°C, and then iso-octanol (11.7 g, 90 mmol) was added through a constant pressure dropping funnel, stirred for 3 hours, warmed to room temperature, and stirred overnight. After traced by TLC that the starting material disappeared, the reaction was stopped. Water and diethyl ether were added for separation and extraction, the diethyl ether solution was collected, washed twice with water and once with saturated brine, dried overnight with anhydrous magnesium sulfate, concentrated and used directly for the next reaction.
[0068] Add isooctyl acetoacetate and acetonitrile (250mL) obtained in the previous step into a 500mL egg-shaped flask, stir in an ice bath for 15 minutes, add triethylamine (18.9mL, 135mmol), and then add methanesul...
Embodiment 2
[0073]
[0074] Under ice-bath conditions, 10-undecen-1-ol (11.6mL, 60mmol) and anhydrous pyridine (30mL) were added into a 100ml egg-shaped flask, and after stirring and mixing to dissolve each other, p-toluenesulfonyl chloride (13.75g , 72 mmol), after stirring in an ice bath for 6 hours, TLC plate tracking showed that the reaction was basically complete (PE: EA = 6: 1, potassium permanganate color). Filter, add 100ml ether to the filtrate, and wash the ether solution with 2N hydrochloric acid (100mL*4). anhydrous sodium sulfate, and dry the organic layer. Most of the ether was spun off, separated by column chromatography, the developer was PE:EA=10:1, and 15.26 g of the product was obtained with a yield of 79%.
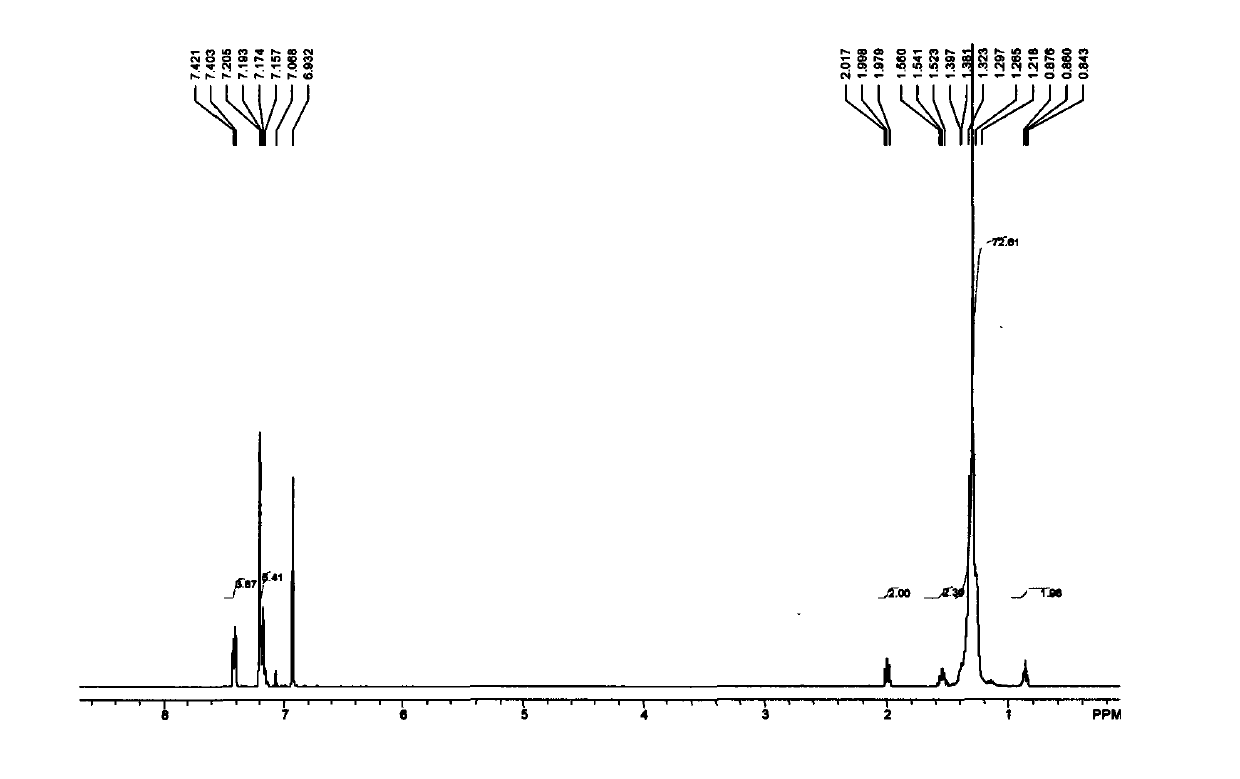
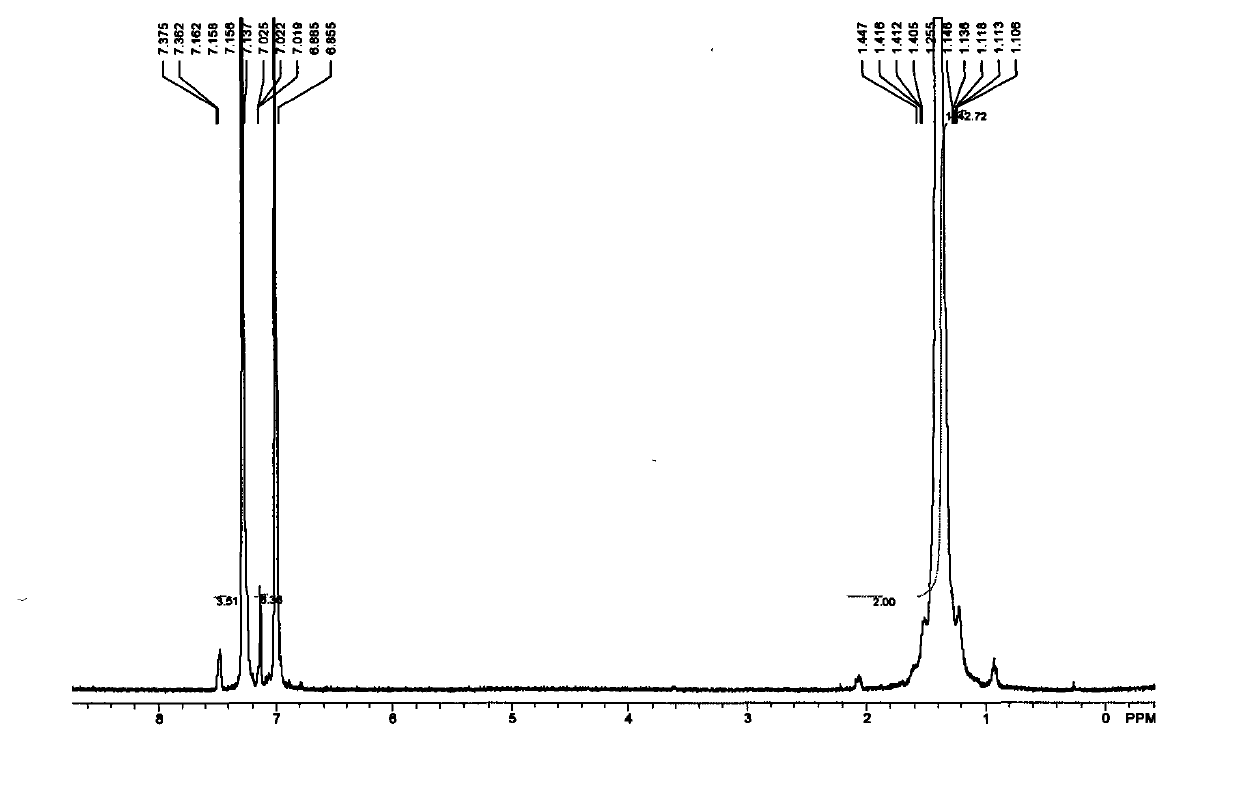
[0075] 1 H NMR (300MHz, CDCl 3 / TMS): δ7.79(d, J=7.8Hz, 2H), 7.34(d, J=8.4Hz, 2H), 5.87-5.73(m, 1H), 5.02-4.91(m, 2H), 4.02( t, J = 6.3 Hz, 2H), 2.45 (s, 3H), 2.06-2.00 (m, 2H), 1.65-1.57 (m, 2H), 1.39-1.23 (brs, 12H).
[0076] Ph 3 As+Li→Ph 2 AsLi
[00...
Embodiment 3
[0082] Pretreatment of arsine monomer:
[0083] Add monomer (5.01g, 13.2mmol) to the stopcock bottle, replace the nitrogen, add toluene (18ml), cool in a dry ice-acetone bath, add triisobutylaluminum (4mL, 16mmol) dropwise to form a 0.5M solution, Stir overnight and warm to room temperature naturally.
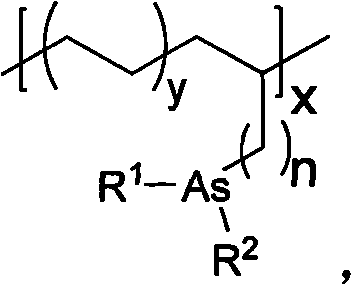
[0084] Preparation of supported catalyst PE-1:
[0085]
[0086] The dry Schlenk bottle was replaced with ethylene gas three times. Under ethylene flow, toluene (70 mL) was added, and the temperature of the oil bath was controlled to 30° C. and stirred for 10 minutes, so that the solution reached the specified temperature and was saturated with ethylene. Triisobutylaluminum-protected arsine monomer (0.5M, 24mL), stirred for 2 minutes, added cocatalyst (MMAO) (1.9M, 15.0mL), stirred for 2 minutes. A toluene solution (3.5 μmol / ml, 8.0 mL) of the metal catalyst was added and the timer was started simultaneously. After 30 minutes of polymerization, the flow of ethylene was q...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com