Anti-H5N1 type bird flue virus vicuna VHH heavy chain antibody as well as preparation method and application thereof
An avian influenza virus and heavy chain antibody technology, applied in the biological field, can solve the problems of low detection sensitivity of antigen components, missed diagnosis, and no very mature products, etc., and achieve the effect of improving early diagnosis and clinical treatment level and high binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
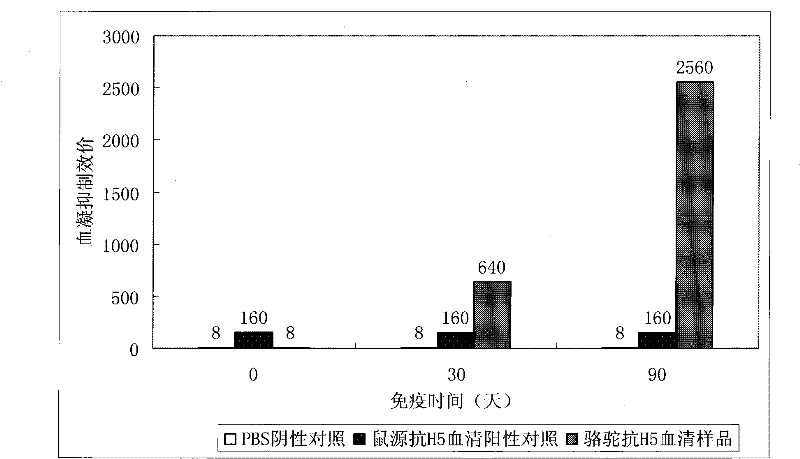
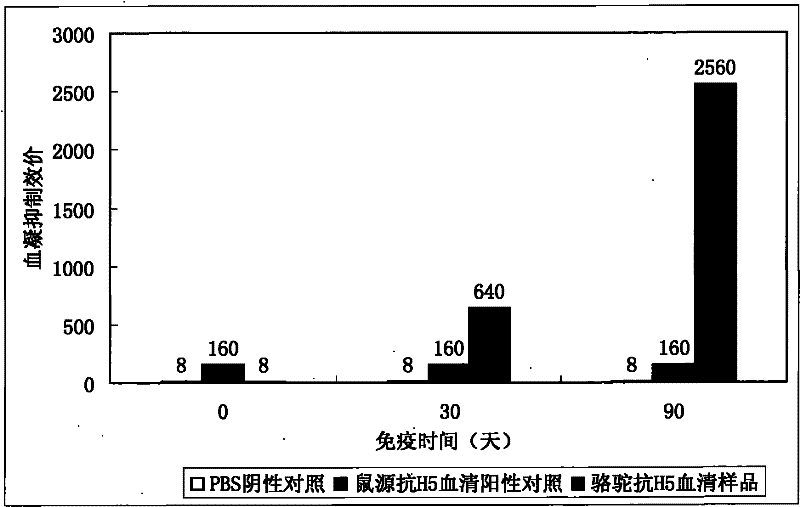
[0038] Example 1: Vicuna immunity and detection of hemagglutination inhibition titer
[0039] 1. Immunization method: Subcutaneous and intramuscular multi-point injection of vaccine (recombinant avian influenza virus inactivated vaccine: H5N1 subtype, Re-1 strain; Harbin Binveken Biotechnology Development Company) into multiple lumps on the back of the neck, follow up and observe the subcutaneous Inject the absorption of the mass to confirm that the immunization is correct.
[0040] 2. Immunization cycle:
[0041] (1) First immunization: the vaccine injection volume is between 40-120mL;
[0042] (2) Second immunization: one month later, collect antiserum samples and determine the titer; carry out the second immunization, the dose is half of the first;
[0043] (3) The third immunization: collect antiserum samples four weeks later, and measure the titer; and carry out the third immunization, the dose is 1 / 4 of the first time; collect antiserum samples four weeks later, and me...
Embodiment 2
[0055] Example 2: Lymphocyte Separation Process
[0056] 1. Anesthetized animals: before anesthesia, inject 1.0 mL of atropine hydrochloride (Tianjin Pharmaceutical Group Xinzheng Co., Ltd.) into the femoral muscle; inject 1.0 mL of Sumianxin injection (Changchun Military University Veterinary Research Institute) into the femoral muscle, and there is a response within 5 minutes. The effective time of anesthesia is 30 minutes; if it cannot wake up naturally, inject 1 mL of Lu Xingning (Changchun Military University Veterinary Research Institute) to wake up.
[0057] 2. Prepare for blood collection: fast for 12 hours (to prevent vomiting after anesthesia, this step can be saved); take 10-20 mL of blood from the jugular vein, store it at room temperature and away from light.
[0058] 3. Blood sample collection
[0059] A. Non-anticoagulant blood: 5mL blood sample, divided into 5 EP tubes; place it in the dark at room temperature for 2 hours, then place it in a refrigerator at 4°...
Embodiment 3
[0068] Example 3: Extraction of total cellular RNA
[0069] 1. Every 2×10 7 Add 1-2mL Trizol (Invitrogen Company, a new type of total RNA extraction reagent to the cells, which can directly extract total RNA from cells or tissues. It contains phenol, guanidine isothiocyanate and other substances, which can quickly break cells and inhibit the release of cells. nuclease), pipette evenly and mix on a vortex shaker for 30 seconds; let stand at room temperature for 10 minutes (for tissue samples, 1 mL of Trizol can be added per 100 mg of tissue, liquid nitrogen grinding or electric homogenizer fully break up the tissue pieces).
[0070] 2. Add about 1 / 5-2 / 5 volume of chloroform (Sigma company), manually shake up and down vigorously for about 1 minute, and let it stand at room temperature for 5 minutes.
[0071] After centrifuging at 12000rpm for 15min at 3.4°C, carefully remove the supernatant (approximately no more than 3 / 5 of the volume of Trizol), transfer the supernatant to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com