Spina gleditsiae extract hydrogel patch and preparation method thereof
A technology of saponin thorn extract and hydrogel, which is applied in the direction of drug combination, medical formula, plant raw materials, etc., can solve the problems that harmful components cannot be separated, harmful metals cannot be completely removed, and the limitation of nano-Chinese medicine, etc., to achieve anti-infective drugs Good efficacy and activity, save raw materials of traditional Chinese medicine, and simple prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 saponins thorn extract hydrogel plaster
[0027] 1. Preparation of Saponaria thorn extract
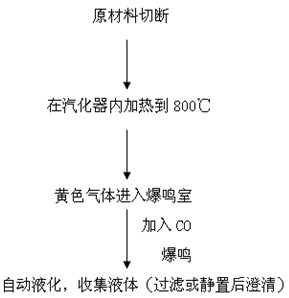
[0028] The preparation of saponin thorn gasification explosive solution is prepared by the reference "Preliminary study on the external antibacterial effect and components of saponin thorn gasification explosive solution" (Journal of Guangdong Pharmaceutical University, 2010, 26 (6): 579~582). Cut the raw material of saponin thorn into sections, and put it in a gasifier (name of equipment: gasification explosion extraction equipment, manufacturer: Tonghua Institute of Traditional Chinese Medicine, Jilin Province, patent name of this equipment: a special device for extracting effective parts of Chinese herbal medicine, Patent No. 01212112.6) is heated to 800°C to produce yellow gas that enters the detonation chamber, and then passes through CO detonation chamber. After automatic liquefaction, the liquid is collected (filtered or clarified after s...
Embodiment 2
[0064] Example 2 Skin irritation test of single administration in rabbits
[0065] 4 New Zealand rabbits were used for animal experiments to remove the skin and hair on the back of the rabbits with a depilatory liquid, with a range of 5cm x 5cm on the left and right sides, and kept them for 24 hours. The medicated patch and blank patch prepared in Example 1 were respectively applied to the depilatory areas on both sides, with a size of 3 cm × 3 cm, fixed with non-irritating bandages, and kept in separate cages. After 24 hours, remove the blank patch and the medicated patch, clean the skin with warm water, observe and record whether there is erythema and edema at the application site 1 hour, 24 hours, 48 hours and 72 hours after removing the drug. The degree of erythema and edema is evaluated from 0 to 4 points, and the smaller the score, the lower the skin irritation.
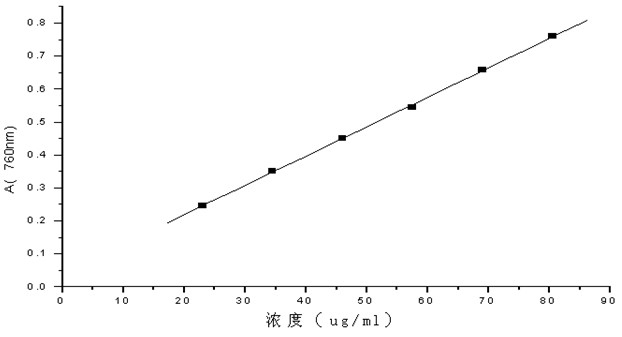
[0066] After a single administration, the skin of the rabbits had no erythema and edema, and the total ...
Embodiment 3
[0067] Example 3 Pharmacodynamic experiment of mice infected with Staphylococcus aureus
[0068] (1) Cultivation and preparation of bacterial strains for infection: ATCC6538 Staphylococcus aureus strains were shaken and grown overnight in nutrient agar medium, and transferred to new nutrient agar medium on the second day. When the bacteria grew to the logarithmic growth phase, use Quickly wash 2 times with sterile normal saline, and then resuspend the bacteria to 1×10 9 CFUs / 100μl or 1×10 8 CFUs / 50 μl.
[0069] (2) Establishment of the mouse skin infection model: the mice were depilated on the back 24 hours before the experiment, and 0.2ml / mouse of Staphylococcus aureus was injected subcutaneously. After 24 hours, purulent infection of the back skin of the mice could be seen.
[0070] (3) Experimental grouping and administration: 40 Kunming mice, half male and half male, were randomly divided into 4 groups, 10 mice in each group. The specifics are as follows: ①medicated p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com