Preparation method of nano positive material for lithium ion battery
A technology for lithium ion batteries and cathode materials, which is applied in the field of nanomaterial preparation technology and green energy, can solve the problems of non-uniformity of material components, long calcination time, complicated preparation process, etc., and achieves easy industrialization amplification and outstanding charge-discharge performance. , the effect of uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
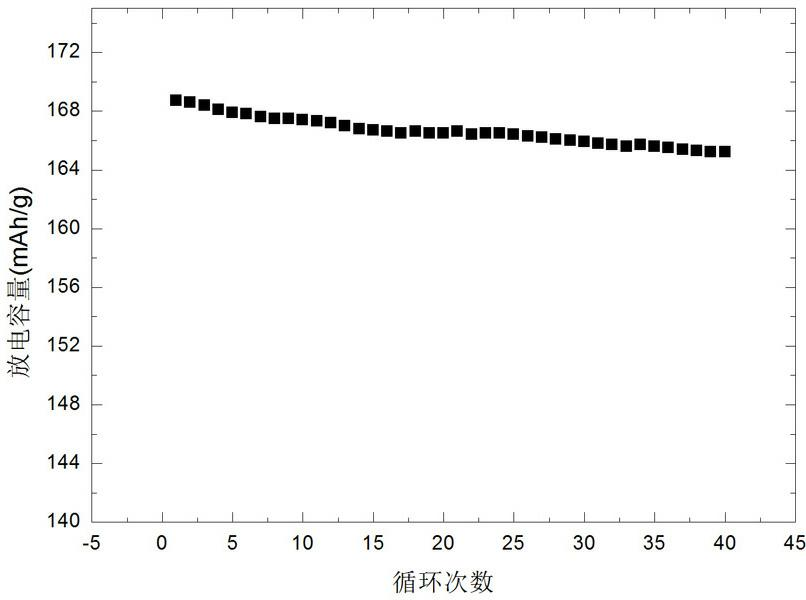
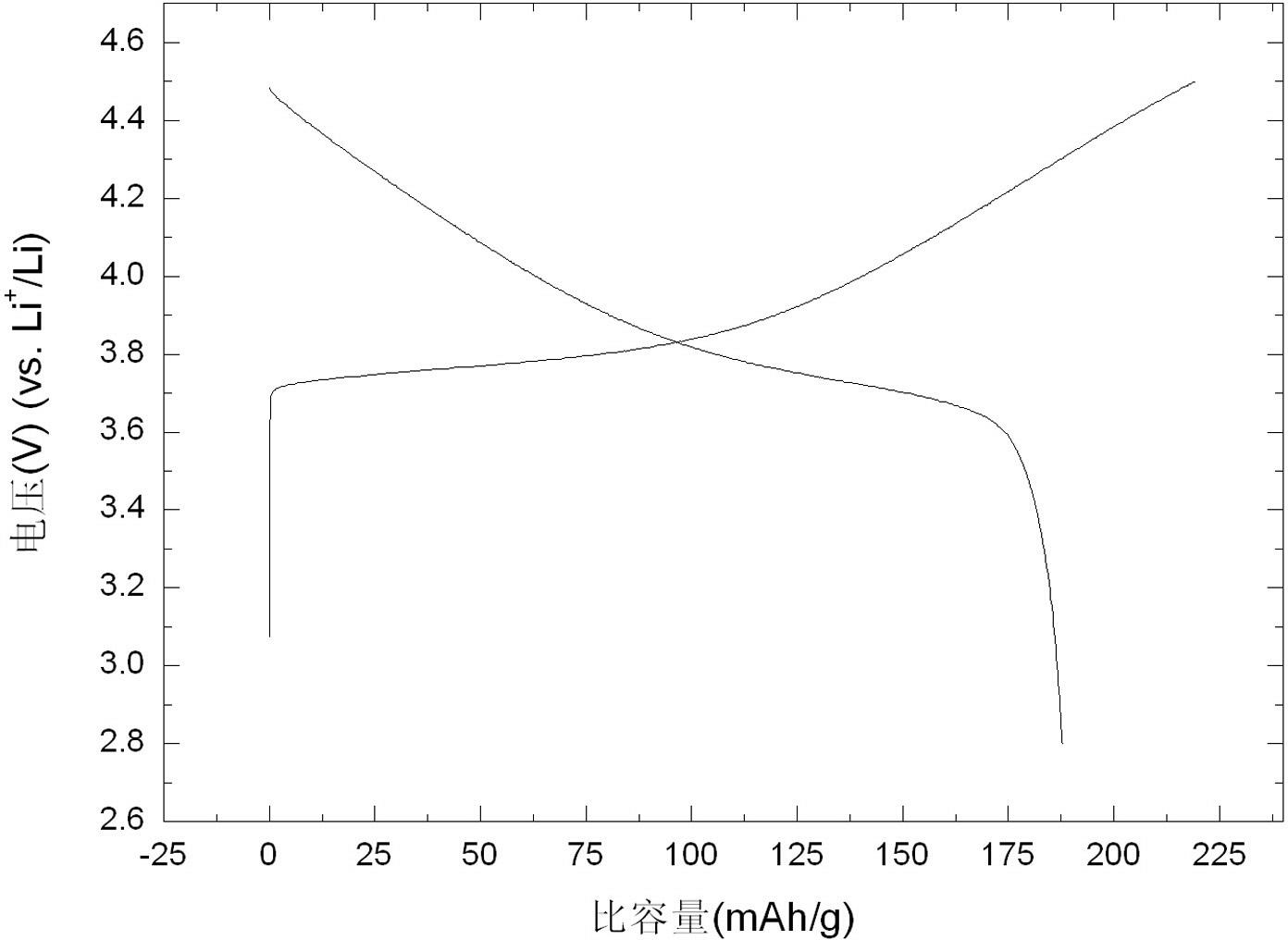
Examples
Embodiment 1
[0028] Embodiment 1: Get 11.63 g nickel nitrate, 7.158 g manganese nitrate and 11.64 g cobalt nitrate, dissolve in deionized aqueous solution, the concentration of metal ion is 2 mol / L, add 0.5 g polypropylene glycol simultaneously, stir 0.5 with 800 rpm Hour. in N 2 Under the atmosphere, 0.08 mol aqueous solution containing lithium hydroxide and 0.08 mol urea aqueous solution were added dropwise to the above solution at the same time, and stirred at 800 rpm, the reaction temperature was kept at 45 °C, and the pH value of the reaction system was kept at 10. Stirring was continued for 2 hours after the precipitate formed. The formed precipitate and solution were transferred to a hydrothermal kettle with a filling degree of 70%. After reacting in a hydrothermal environment at 160 °C for 12 hours, the precipitate was taken out, filtered and cleaned, and the filter cake was dried to obtain the precursor nickel manganese cobalt oxide. The precursor was mixed with 2.76 g lithium n...
Embodiment 2
[0029] Embodiment 2: Get 9.95 g nickel acetate, 9.80 g manganese acetate and 9.96 g cobalt acetate and dissolve in deionized aqueous solution, the concentration of metal ion is 2 mol / L, add 1.5 g carbon nanotubes simultaneously, stir 0.6 with 1000 rpm Hour. in N 2 Under the atmosphere, 0.12 mol aqueous solution containing sodium hydroxide and 0.05 mol urea aqueous solution were added dropwise to the above solution at the same time, and stirred at 1200 rpm, the reaction temperature was kept at 40°C, and the pH value of the reaction system was kept at 11. Stirring was continued for 2 hours after the precipitate formed. The formed precipitate and solution were transferred to a hydrothermal kettle with a filling degree of 70%. After reacting in a hydrothermal environment at 150 °C for 16 hours, the precipitate was taken out, filtered and cleaned, and the filter cake was dried to obtain the precursor nickel manganese cobalt oxide. Mix the precursor with 4.08 g lithium acetate in ...
Embodiment 3
[0030] Example 3: Dissolve 17.06g of nickel oxalate, 19.06g of manganese oxalate, and 19.59g of cobalt oxalate in a deionized aqueous solution. The concentration of metal ions is 2 mol / L. At the same time, 0.7 g of corn stalks are added and stirred at 1000 rpm for 1 hour . in N 2 Under the atmosphere, 0.16 mol aqueous solution containing lithium hydroxide and 0.05 mol urea aqueous solution were added dropwise to the above solution at the same time, and stirred at 1100 rpm, the reaction temperature was kept at 45 °C, and the pH value of the reaction system was kept at 12. Stirring was continued for 2 hours after the precipitate formed. The formed precipitate and solution were transferred to a hydrothermal kettle with a filling degree of 85%. After reacting in a hydrothermal environment at 200 °C for 12 hours, the precipitate was taken out, filtered and cleaned, and the filter cake was dried to obtain the precursor nickel manganese cobalt oxide. Mix the precursor and 4.08 g li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com